Marginal-zone lymphoma (MZL) arises from B-lymphocytes in the marginal zone of lymphoid tissue. This slow-growing indolent B-cell lymphoma represents approximately 12% of all cases of non-Hodgkin lymphoma (NHL) in adults.1 MZL is divided into 3 subtypes, including mucosa-associated lymphoid tissue (MALT), nodal MZL, and splenic MZL.1 MALT lymphoma is the most common of these subtypes and occurs in the stomach, intestines, salivary glands, thyroid, eyes, and lungs.1 In MALT lymphoma, autoimmune processes or chronic infection cause B-cells to accumulate.2,3 Helicobacter pylori is 1 of at least 6 microbial species associated with lymphoproliferation in gastric MALT lymphoma.3
Short-term antibiotic therapy is the initial treatment of gastric MALT lymphoma and is effective in approximately 70% to 90% of patients.1 For relapsed gastric MALT lymphoma, nonsurgical treatment options include chemotherapy, bortezomib (Velcade), low-dose radiation, and rituximab (Rituxan).1
The other types of MZL can appear in several areas of the body, and their treatment is based on the location and the extent of disease spread. Lung, breast, or spleen surgery can be considered, or radiation therapy, with or without chemotherapy.1 First-line chemotherapy regimens for advanced MZL include bendamustine plus rituximab, and the R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen.1
Active surveillance is appropriate for patients with asymptomatic MZL.2 The 5-year overall survival rate for patients with MALT lymphoma exceeds 85% in the majority of the cases.2
Graft-versus-host disease (GVHD) is a serious complication of allogeneic stem-cell transplant, in which the transplanted cells or bone marrow attack the recipient’s body.4 GVHD manifests as acute or chronic disease. The common risk factors for acute GVHD include engraftment of cells or bone marrow from a human leukocyte antigen (HLA)-mismatched related donor, or from an HLA-matched unrelated donor. Other risk factors include a female donor with a history of pregnancy or older age of the donor or the recipient.4
An estimated 30% to 70% of patients who undergo allogeneic transplant have chronic GVHD.5 Chronic GVHD typically begins later after transplant and lasts longer than acute GVHD.6
Common risk factors for chronic GVHD include engraftment of cells or bone marrow from an HLA-mismatched related donor or from an HLA-matched unrelated donor, a history of acute GVHD, and older age.4 Initial symptoms of chronic GVHD typically include skin rash and/or mouth sores.
Historically, the treatment of chronic GVHD was based on prednisone and other anti-inflammatory or immunosuppressive drugs, but no drug was specifically approved by the US Food and Drug Administration (FDA) for this indication.6
Ibrutinib Receives 2 New Indications in 2017
On January 19, 2017, the FDA granted accelerated approval of a new indication for ibrutinib (Imbruvica; Pharmacyclics) for the treatment of patients with relapsed or refractory MZL who require systemic therapy after at least 1 anti-CD20–based therapy.7 This was the first FDA approval of a nonchemotherapy treatment for patients with relapsed or refractory MZL; the approval was based on overall response rate (ORR) data.7
Then on August 2, 2017, the FDA approved ibrutinib for the treatment of adults with chronic GVHD after failure of ≥1 systemic therapies.5 Ibrutinib is the first drug approved by the FDA specifically for chronic GVHD; ibrutinib received breakthrough therapy and orphan drug designations for this indication.5
“Patients with cGVHD who do not respond to other forms of therapy—typically corticosteroids to suppress their immune system—now have a treatment option specifically indicated to treat their condition,”5 said Richard Pazdur, MD, Director of the FDA’s Oncology Center of Excellence.
Ibrutinib was initially approved by the FDA in 2013 for mantle-cell lymphoma, a rare type of NHL, in patients who had received ≥1 therapies.8 In February 2014, ibrutinib was approved for chronic lymphocytic leukemia (CLL) in patients who had received ≥1 therapies, and in July 2014, it was approved for patients with CLL and 17p deletion.8 In 2015, ibrutinib received a new indication for Waldenström’s macroglobulinemia after ≥1 previous therapies.8,9 And in 2016, ibrutinib received new indications as first-line treatment of patients with CLL or with small lymphocytic lymphoma.8
Mechanism of Action
Ibrutinib is a small-molecule inhibitor of Bruton’s tyrosine kinase, a signaling molecule of the B-cell antigen receptor and cytokine receptor pathways.8 Preclinical studies have demonstrated that ibrutinib prevents the activation of downstream pathways affected by Bruton’s tyrosine kinase, inhibits cell proliferation, and promotes apoptosis of cancer cells.10 Ibrutinib is the first FDA-approved drug designed to target Bruton’s tyrosine kinase, a protein necessary for the growth and survival of B-cells.8
Dosing and Administration
In February 2018, the FDA approved a once-daily dosing schedule of ibrutinib. Ibrutinib is available as 70-mg and 140-mg capsules and as 140-mg, 280-mg, 420-mg, and 560-mg tablets.8 In patients with MZL, the recommended dose of ibrutinib is 560 mg orally once daily. Dose reduction recommendations are listed in the prescribing information. In patients with chronic GVHD, the recommended dosing is 420 mg taken orally once daily. Ibrutinib should be administered orally at the same time each day, and should be swallowed whole with water. The capsule should not be opened, broken, or chewed. The tablet should not be cut, crushed, or chewed.8
Pivotal Clinical Trial
Marginal-Zone Lymphoma
The approval of ibrutinib for MZL was based on the phase 2, open-label, multicenter PCYC-1121 clinical trial of 63 patients with previously treated MZL.11 This study involved patients with MALT (N = 32), nodal MZL (N = 17), or splenic MZL (N = 14). The primary end point was ORR, as assessed by an independent review committee.11 The majority (92%) of patients (median age, 66 years) had an Eastern Cooperative Oncology Group performance status 0 or 1; were female (59%) and white (84%). The median time since MZL diagnosis was 3.8 years; patients received a median of 2 previous therapies (range, 1-9) for MZL.11
After a median follow-up of 19.4 months, the ORR was 46% (95% confidence interval [CI], 33.4-59.1; Table 1).8 The duration of response was not reached at the time of the FDA approval, and ranged from 16.7 months to “not reached.” The response rates were 46.9% for MALT lymphoma; 41.2% for nodal MZL; and 50% for splenic MZL.8
Chronic GVHD
The approval of ibrutinib for chronic GVHD was based on data from the open-label, multicenter, single-arm clinical trial PCYC-1129-CA, which included 42 patients with chronic GVHD that failed after first-line corticosteroid therapy. The majority of patients (median age, 56 years) were male (52%) and Caucasian (93%). The most common malignancies leading to transplant were various types of leukemia.8,12
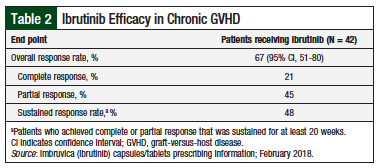
At a median follow-up of 13.9 months, the best ORR (the primary end point) was 67% (95% CI, 51-80); the median time to response was 12.3 weeks.12 Overall, 48% of responders had a sustained response, defined as complete or partial response that was sustained for at least 20 weeks (Table 2).8
Adverse Reactions
The most common (≥20%) adverse reactions in patients with MZL were thrombocytopenia (49%), fatigue (44%), diarrhea (43%), bruising (41%), hemorrhage (30%), anemia (43%), rash (29%), peripheral edema (24%), arthralgia (24%), neutropenia (22%), upper respiratory tract infection (21%), cough (22%), dyspnea (21%), musculoskeletal pain (40%), and nausea (25%).8
Grade ≥3 adverse events were reported in 67% of patients, with anemia, pneumonia, and fatigue being the most common events.11 Diarrhea was the most common (3%) event leading to ibrutinib discontinuation in patients with MZL.8
The most common (≥20%) adverse events in patients with chronic GVHD were fatigue (57%), bruising (40%), diarrhea (36%), thrombocytopenia (33%), muscle spasms (29%), stomatitis (29%), nausea (26%), hemorrhage (26%), anemia (24%), and pneumonia (21%). Overall, 24% of patients with chronic GVHD discontinued ibrutinib treatment because of side effects. The most common adverse reactions leading to discontinuation were fatigue and pneumonia.8
Ibrutinib has no contraindications.8
Drug Interactions
Ibrutinib should not be used with strong or moderate cytochrome (CY) P3A inhibitors or with strong CYP3A inducers, and its dose should be reduced if a moderate CYP3A inhibitor must be used.8
Use in Specific Populations
Because ibrutinib can cause fetal harm, women of reproductive potential should avoid pregnancy while taking ibrutinib and for up to 1 month after ending treatment. Men should avoid fathering a child while taking ibrutinib and for up to 1 month after ending treatment.8
Anemia and grade ≥3 pneumonia have been reported more often in older than young patients who used ibrutinib.8
Exposure to ibrutinib increases with liver failure and is not recommended for patients with moderate or severe liver disease. Patients should be monitored for liver toxicity.8
Select Warnings and Precautions
Bleeding events of any grade occurred in approximately 50% of patients who received ibrutinib. Because ibrutinib can increase hemorrhage risk in patients receiving antiplatelet or anticoagulant therapies, patients should be monitored for bleeding.8
Fatal and nonfatal infections, including progressive multifocal leukoencephalopathy, have occurred in clinical trials of ibrutinib.8
Severe cytopenias were reported with ibrutinib. Complete blood cell counts should be monitored monthly.8
Atrial fibrillation and atrial flutter have occurred in 6% to 9% of patients who received ibrutinib. If persistent atrial fibrillation is noted, dose modification or discontinuation of ibrutinib should be considered.8
Second malignancies, including nonskin carcinomas, have occurred with ibrutinib. Nonmelanoma skin cancer is the most common second malignancy reported.8
Patients should be monitored closely for tumor lysis syndrome.8
Conclusion
Ibrutinib, a first-in-class inhibitor of Bruton’s tyrosine kinase, is the first drug approved in the United States for patients with relapsed or refractory MZL, a rare and indolent subtype of NHL. Ibrutinib is also the first drug approved by the FDA specifically for patients with chronic GVHD, which can occur after allogeneic transplant, providing a new treatment option for those 2 patient populations who do not respond to other systemic therapies.
References
- Lymphoma Research Foundation. Marginal zone lymphoma. www.lymphoma.org/site/pp.asp?c=bkLTKaOQLmK8E&b=6554677. Accessed May 22, 2017.
- Zinzani PL. The many faces of marginal zone lymphoma. Hematology Am Soc Hematol Educ Program. 2012;2012:426-432.
- Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127:2082-2092.
- Cleveland Clinic Foundation. Graft vs host disease: an overview in bone marrow transplant. November 27, 2017. https://my.clevelandclinic.org/health/diseases/10255-graft-vs-host-disease-an-overview-in-bone-marrow-transplant. Accessed March 18, 2018.
- US Food and Drug Administration. FDA approves Imbruvica (ibrutinib) for chronic graft versus host disease. August 2, 2017. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm569711.htm. Accessed November 30, 2017.
- Chronic Graft Versus Host Disease Consortium, Rare Diseases Clinical Research Network. Chronic graft-versus-host disease. www.rarediseasesnetwork.org/cgvhd/chronic/. Accessed November 30, 2017.
- US Food and Drug Administration. FDA approves Imbruvica (ibrutinib) as first treatment specifically indicated for relapsed/refractory marginal zone lymphoma (MZL). January 19, 2017. Accessed May 22, 2017.
- Imbruvica (ibrutinib) capsules/tablets [prescribing information]. Sunnyvale, CA: Pharmacyclics; Horsham, PA: Janssen Biotech; February 2018.
- US Food and Drug Administration. FDA expands approved use of Imbruvica for rare form of non-Hodgkin lymphoma: first drug approved to treat Waldenström’s macroglobulinemia. Press release. January 29, 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm432123.htm. Accessed May 22, 2017.
- Akinleye A, Chen Y, Mukhi N, et al. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol. 2013;6:59.
- Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129:2224-2232.
- Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243-2250.