The impact of chemotherapy-induced nausea and vomiting (CINV) for the patient with cancer cannot be overemphasized. Uncontrolled CINV has been cited as one of the greatest fears among people undergoing cancer treatments.1,2 Advances in antiemetic therapy in the past 20 years have improved patient experiences and outcomes. Despite this, the majority of patients with cancer will experience CINV during their chemotherapy treatments.1,3-6 In particular, nausea is less well controlled than vomiting. Uncontrolled CINV symptoms lead to reduced quality of life, decreased ability for self-care, and low patient morale, as well as to increased healthcare costs.4-7 Preventing and controlling CINV is a vital part of the pharmaceutical care of the patient with cancer. This article reviews the current therapies available for the prevention and treatment of CINV.
Neurophysiology
CINV represents a wide range of symptoms, from mild, queasy nausea to repetitive vomiting and retching. Acute symptoms can begin within minutes of the administration of chemotherapy, but delayed symptoms can also last for many days afterward. Anticipatory CINV can also occur before chemotherapy administration, as a result of a conditioned response to poor emetic control in previous cycles of chemotherapy. Acute CINV is described as symptoms occurring within the first 24 hours after chemotherapy. Delayed CINV is experienced by patients after the acute phase; these nausea and vomiting symptoms usually peak at about 2 to 3 days after chemotherapy but may last for several days longer.
In addition to chemotherapy, many other medical conditions can lead to nausea and vomiting in patients with cancer. These include hypercalcemia, gastroparesis, gastrointestinal reflux, brain metastases, infections, and many others. Many medications used by patients with cancer can lead to nausea and vomiting, including anti biotics, antifungals, opiate analgesics, and others. It is important to consider these other causes and treat them appropriately, even though they may coincide with CINV.
The symptoms of CINV result from activation of parts of the central nervous system (CNS) and the peripheral nervous system. In the classically described pathway, the toxin exposure is detected by the chemoreceptor trigger zone in the CNS, as well as via the enterochromaffin cells in the gastrointestinal tract. In addition, signals from the cerebral cortex, the limbic system, and the vestibular systems can trigger or accentuate the emetic response. In reaction to these signals, the vomiting center of the CNS activates the emetic response, which includes salivation, contraction of the abdominal muscles, relaxation of the esophageal sphincter, and contraction of the stomach muscles, as well as tachycardia, dizziness, and sweating.
The transmission of the vomiting signals seen with CINV involves multiple neurotransmitters and receptors. The predominant receptors are the serotonin (5-hydroxytryptamine) type-3 (5-HT3) receptor antagonists, neurokinin-1 (NK1) antagonists, and dopamine receptors. Additional neurotransmitters involved include corticosteroid, endogenous cannabinoids, GABA, acetylcholine, and histamine. The activation or inhibition of these neurotransmitters forms the basis of pharmacologic therapy for CINV. Because multiple neurotransmitters are involved in this process, multiple antiemetic medications are necessary for the maximal prevention and treatment of CINV.
Risk Factors
The risk for CINV varies among patients who receive chemotherapy. Patient-related risk factors as sociated with higher rates of CINV include female sex, younger age, poor emetic control in previous chemotherapy cycles, a history of motion sickness or nausea during pregnancy, anxiety and/or depression, and no history of alcohol abuse.
The primary determinant of the prevalence of CINV is the inherent emetogenicity of the chemotherapy agents administered. The emetogenicity of chemotherapy agents has been classified into 4 categories.7,8 Highly emetogenic agents are those causing CINV in <90% of patients. The largest group is the moderately emetogenic agents, leading to CINV in 30% to 90% of patients. Low emetogenic level chemotherapy results in CINV symptoms in 10% to 30% of patients. Finally, agents with minimal emetogenicity cause CINV symptoms in fewer than 10% of patients.7,8
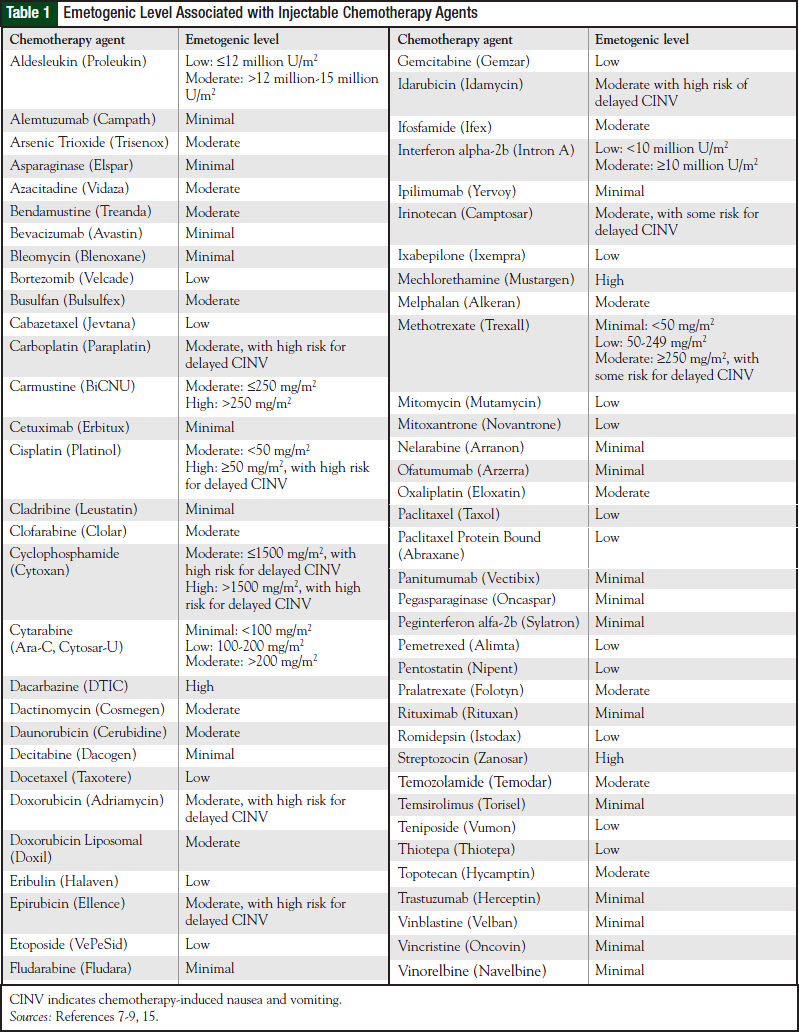
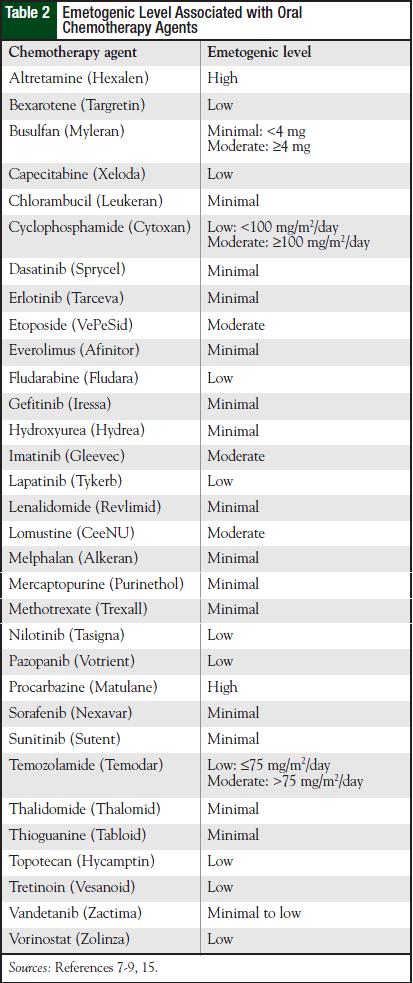
Table 1 lists the emetogenicity of various chemotherapy agents given by injection. The characteristics of CINV seen with oral chemotherapy agents are different with regard to onset, duration, and severity.8 Most oral chemotherapy agents have minimal to low emetogenicity, but some have moderate to high emetogenicity. Table 2 lists the emetogenic level seen with oral chemotherapy agents.
In addition to the emetogenicity, other chemotherapy- related risk factors include high doses, fast infusions, and multiday chemotherapy administration. Multiday chemotherapy regimens are particularly challenging, because the delayed CINV symptoms are overlaid on the latter day’s acute CINV. Because most chemotherapy regimens use more than 1 antineoplastic agent given on a single day, it is difficult to predict the emetogenicity of these combination regimens. It is recommended that the antiemetic regimen be designed to be consistent with the highest emetogenicity associated with the chemotherapy agents given on each day.
The 5-HT3 Antagonists
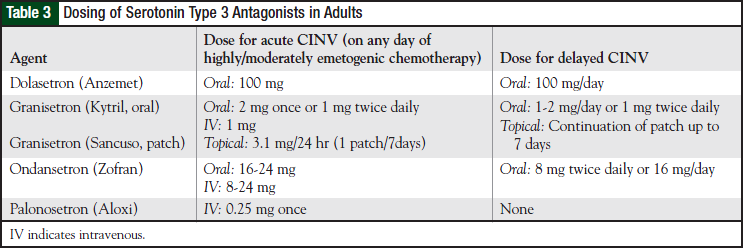
The 5-HT3 receptor antagonists are effective anti - emetic agents associated with minimal adverse effects. These agents block serotonin release from the gastrointestinal tract in addition to blocking serotonin receptors in the CNS. When initially approved in the early 1990s, the 5-HT3 antagonists became the first highly active antiemetics that did not have substantial adverse effects. The 4 agents in this class—dolasetron, granisetron, ondansetron, and the most recently approved palonosetron—are available in a variety of dosage forms in adults (Table 3).
The 5-HT3 antagonists are more potent as antiemetic agents than antinausea medications. These agents demonstrate a plateau effect: once enough medication has been given to block the receptors, more medication is not more effective. For patients who can take oral medications, oral therapy is as effective as intravenous (IV) dosing.1,7,9,10 Except for palonosetron, the 5-HT3 antagonists are not more effective than other agents (ie, prochlorperazine, aprepitant, or dexamethasone) for the prevention of delayed CINV.1,9-13
The 5-HT3 antagonists are well tolerated and have few adverse effects. The most often reported side effects include headache, constipation, and diarrhea. In addition, prolonged corrected QT (QTc) interval and other cardiac dysrhythmias have been reported, with an incidence of up to approximately 5% (based on Lexicomp and Micromedex information). It is unclear what the true incidence of adverse events is for the different 5-HT3 antagonists, because these are likely underreported.
The US Food and Drug Administration (FDA) has now warned that IV dolasetron should not be used for the prevention of CINV, because the drug increases the risk for torsades de pointes. The FDA has added warnings to the label of ondansetron against the use of the drug by patients with a long QT syndrome and is recommending electrocardiographic monitoring for patients at high risk for this event—those with electrolyte abnormalities, congestive heart failure, or bradyarrhythmias, and patients using concomitant medications that can increase the QTc interval. For patients without underlying cardiac rhythm disorders or concurrent treatment with other medications that prolong the QTc interval, it is not clear how clinically significant this potential adverse effect is.
It is generally accepted that there are no substantial differences in antiemetic efficacy among the 5-HT3 antagonists, except for palonosetron.7,9,10,14,15 With its longer half-life, palonosetron was shown in the original clinical trials to have equivalent or superior effectiveness in the acute and the delayed phases of CINV.7,10,15 These original trials were hampered by including comparator groups that did not reflect the standard of care for highly or moderately emetogenic chemotherapy.
Since then, several studies have provided information that further delineates the effects of palonosetron.16-20 When taken in total, current evidence suggests that palonosetron has a small, but real, increase in antiemetic efficacy, especially in the delayed phase.7,16-20 In light of this new evidence, the National Comprehensive Cancer Network (NCCN) guidelines now list palonosetron as the preferred 5-HT3 antagonist for highly and moderately emetogenic chemotherapy.7
The antiemetic guidelines from the Multinational Association of Supportive Care in Cancer (MASCC), as well as the guideline from the American Society of Clinical Oncology (ASCO), identify palonosetron as the preferred 5-HT3 antagonist for moderately emetogenic chemotherapy that is not based on anthracyclinecyclophosphamide regimens.9,15 Palonosetron, however, is substantially more expensive than the alternative agents, and cost-effectiveness should be taken into consideration when choosing among the available 5-HT3 receptor antagonists.
Corticosteroids
Corticosteroids, and dexamethasone in particular, have long been used for the prevention and treatment of CINV. Despite the widespread use of corticosteroids, their precise antiemetic mechanism of action is still unclear. The potential mechanisms may include activation of glucocorticoid receptors in the CNS, decreased release of serotonin, inhibition of prostaglandin synthesis in the cerebral cortex, and alteration of cortical input into the emetic center in the CNS.21
Although dexamethasone can be used alone for a chemotherapy regimen with low emetogenicity, it is more often used in conjunction with a 5-HT3 antagonist, with or without an NK1 receptor antagonist, for moderately to highly emetogenic regimens. In these situations, dexamethasone adds about 15% to 20%21 to the complete antiemetic response rate. Dexamethasone is active in the acute and the delayed phases of CINV.
The short-term use of dexamethasone in doses used in CINV is usually well tolerated, although the drug is sometimes underutilized, because of concern for its associated adverse effects.21 The most frequently reported adverse effects include insomnia, anxiety, mood changes, increased appetite, mild fluid retention, stomach discomfort, hyperglycemia, and a burning in the rectal/vaginal area when IV doses are infused too rapidly.
Hyperglycemia is often seen in patients with preexisting or undiagnosed diabetes. In some patients, the hyperglycemic effect is significant enough to warrant additional glucose monitoring or alterations of antidiabetic medications.
The appropriate dexamethasone dose depends on the emetogenicity of the chemotherapy and on whether an NK1 antagonist is coadministered. One group of researchers explored the dose–response relationship of dexamethasone in the context of highly emetogenic chemotherapy, showing that the 12-mg and 20-mg doses of the drug were associated with a higher complete response rate than the 4-mg and 8-mg doses.22 The investigators also studied different doses and regimens of dexamethasone in moderately emetogenic chemotherapy and found that higher doses were not more effective than a single 8-mg dose.23
These studies have helped shape the current dosing recommendations found in the various guidelines. The dose and schedule of dexamethasone should be modified if a patient will also receive an NK1 antagonist. Aprepitant and fosaprepitant inhibit the cytochrome (CY) P450 3A4–based metabolism of dexamethasone and result in an approximately 2-fold increase in the area under the curve (AUC) of dexamethasone. Overall, dexamethasone doses are reduced by half if used concurrently with aprepitant.
Available CINV guidelines differ slightly in their recommendations for dexamethasone dose.7,9,15 Most guidelines recommend 12 mg to 20 mg of dexamethasone for the acute phase of highly emetogenic chemotherapy and 8 mg to 12 mg for moderately emetogenic chemotherapy. For regimens with a high risk of delayed CINV, oral dexamethasone 8 mg daily or twice daily for 2 to 3 days is recommended, depending on whether an NK1 antagonist is also given.7,9,15
Newer research has explored the question of whether, when administered with palonosetron, a single dose of dexamethasone offers the same efficacy as the typical 3-day dexamethasone regimen.24,25 Two noninferiority studies enrolled patients receiving moderately emetogenic chemotherapy. All 632 patients in the 2 studies combined received IV palonosetron 0.25 mg and IV dexamethasone 8 mg before chemotherapy administration. The patients in the control arms of both studies also received oral dexamethasone on days 2 and 3 (8 mg daily in one study and 4 mg twice daily in the other study).24,25
Both trials showed that the 1-day dexamethasone regimen was noninferior to the 3-day regimen for patients receiving moderately emetogenic chemotherapy. How - ever, in one study, the benefit was most apparent in patients receiving chemotherapy other than anthracycline- cyclophosphamide regimens, and in the other study there was a trend toward better nausea control on day 3 with the 3-day dexamethasone regimen.24,25
Neurokinin-1 Antagonists
The NK1 antagonists block the action of substance P on the emetic pathways. Aprepitant is the NK1 antagonist administered orally. Fosaprepitant is a prodrug of aprepitant, administered intravenously. Early trials of aprepitant showed that aprepitant administration significantly increased the complete antiemetic response by 12% to 20%, depending on the trial.26 In these trials, aprepitant was administered orally (125 mg before chemotherapy and 80 mg on days 2 and 3), along with a 5-HT3 antagonist and dexamethasone, in patients receiving moderately and highly emetogenic chemotherapy.26
Aprepitant and fosaprepitant were very well tolerated in these trials, and adverse effects did not differ between the 2 treatment arms.26 Venous irritation has been noted with IV administration, which may require dilution in a larger volume of IV solution. Aprepitant/fosaprepitant is recommended for patients receiving highly emetogenic chemotherapy, as well as those receiving moderately emetogenic chemotherapy associated with a high risk for delayed nausea and vomiting.
Aprepitant is metabolized primarily via the CYP3A4 pathway and to a lesser degree via CYP1A2 and CYP2C19 pathways. The activity of aprepitant may be altered when administered with medications that are CYP3A4 inhibitors or inducers. Aprepitant itself is a moderate inhibitor of CYP3A4 and may affect the concentrations of other agents metabolized by this pathway.26,27 The results of clinical trials and clinical observation do not reveal any clinically relevant drug interactions with chemotherapy agents metabolized via the CYP3A4 pathway. One important drug interaction, however, is seen with corticosteroids. Dexamethasone is metabolized via the CYP3A4 pathway, and roughly a 2-fold increase in AUC is seen when dexamethasone is administered with aprepitant, although this effect is greater with oral dexamethasone than with IV doses of the drug.26, 27
In addition, aprepitant is an inducer of the CYP2C9 pathway, and it has effects on the elimination of the S-isomer of warfarin, which is metabolized by the CYP2C9 pathway.26, 27 Approximately 5 days after completion of aprepitant therapy a reduction is seen in the S-isomer concentration, with a concurrent decrease in the international normalized ratio (INR). Because aprepitant is used intermittently, the INR value should be monitored closely for 2 weeks after each dose or cycle of aprepitant or fosaprepitant.
To enable IV administration of the first dose of an NK1 antagonist before chemotherapy, fosaprepitant was developed as an IV prodrug of aprepitant. A pharmacokinetic analysis showed that a 115-mg IV dose of fosaprepitant is equivalent to that of a 125-mg oral dose of aprepitant.28
Increasing evidence shows that the initial dose of an NK1 antagonist provides most of the antiemetic activity of aprepitant or of fosaprepitant. A recent trial demonstrated the effectiveness of a single 150-mg dose of fosaprepitant in the prevention of CINV.29 In this large study of 2247 patients receiving highly emetogenic chemotherapy, one group received fosaprepitant IV 150 mg before chemotherapy and the other group received the typical aprepitant oral regimen of 125 mg, 80 mg, and 80 mg over 3 days. All patients received ondansetron and dexamethasone, although patients in the fosaprepitant arm received more dexamethasone on days 3 and 4, because of the expectation that the drug interaction would have dissipated. The complete emetic response in the fosaprepitant arm was found to be noninferior to that seen in the aprepitant arm (71.9% vs 72.3%, respectively).29
Other Antiemetics The group consisting of the 5-HT3 antagonists, dexamethasone, and the NK1 antagonists define the most active antiemetic medications, but there is still a need for alternative antiemetics. In general, it is best to consider agents with a different pharmacologic mechanism of action. Dopamine antagonists, such as prochlorperazine or promethazine, are often used for breakthrough CINV symptoms. The butyrophenones (eg, haloperidol, droperidol) are highly active antiemetics but can cause significant sedation and have the potential to prolong the QTc interval.
The benzodiazepine lorazepam is frequently used for the treatment of breakthrough nausea and vomiting. Exactly how lorazepam acts as an antiemetic is unclear, but it likely affects the limbic or cortical input into the vomiting center. Its additional activity in reducing anxiety is an added benefit. When low-to-moderate doses (ie, 0.5-1.0 mg) are used, excessive sedation is not usually a problem.
Cannabinoids can be useful adjunctive treatments for CINV in selected patients. Dronabinol, and more recently nabilone, are orally available cannabinoid agents. These medications have substantial adverse effects, such as sedation, dysphoria or euphoria, and dry mouth, as well as other CNS adverse effects. These agents are usually reserved for patients who have an inadequate response to other antiemetics and who can tolerate the associated adverse effects.
Olanzapine was originally developed as an atypical antipsychotic medication but was found to have antiemetic activity thought to be caused by its inhibitory activity at various serotonin and dopamine receptors. After early experience with olanzapine in the treatment of nausea and vomiting in hospice patients, noncontrolled studies of olanzapine have shown the drug’s activity in conjunction with 5-HT3 antagonists and dexa - methasone in CINV.
A recent comparison study showed that a group of patients receiving olanzapine (along with a 5-HT3 antagonist and dexamethasone) had a substantially improved complete emetic response rate compared with patients who did not receive olanzapine.30 The most frequently reported adverse effects with olanzapine include drowsiness, dry mouth, and dizziness. Olanzapine is also highly active in relieving breakthrough CINV symptoms in patients with highly refractory symptoms and is perhaps most often used in this situation.31,32
Gabapentin also has been studied in the prevention of nausea and vomiting. Early research with this agent began after the drug’s antiemetic action was noticed in patients with breast cancer who were receiving the drug to help relieve hot flashes.
After showing activity in the prevention of CINV in noncontrolled trials, gabapentin was studied in a randomized, double-blind fashion in 8 patients.33 The gabapentin dose in the experimental arm was titrated upward 5 days before chemotherapy began and continued until 5 days after chemotherapy. Patients in both arms received standard therapy with ondansetron and dexamethasone. Significantly more patients had complete antiemetic response in the treatment arm than in the control arm, and the gabapentin therapy was well tolerated.33 More research will help to define the role of gabapentin in the prevention of CINV.
Treatment Regimen Recommendations
Consensus and evidence-based guidelines and recommendations have been published from 3 major professional oncology groups. The guideline from ASCO was updated in 2011.9 Recommendations from the MASCC and the European Society of Medical Oncology were updated and published in 2011.15 The guidelines from the NCCN are revised and electronically published frequently, often multiple times annually, as new information becomes available.7
Although there are some differences between the guidelines, they largely agree on the basic framework of recommended antiemetic treatment of CINV.7,9,15 The prevention of acute CINV in patients receiving highly emetogenic chemotherapy should include a 5-HT3 antagonist and dexamethasone, along with an NK1 antagonist. For the prevention of delayed CINV in those receiving highly emetogenic chemotherapy, dexamethasone and an NK1 antagonist (if the IV 150-mg fosaprepitant dose is not used) is recommended.7,9,15
For cases with moderately emetogenic chemotherapy, patients should receive a 5-HT3 antagonist and dexamethasone, with or without an NK1 antagonist, in the acute phase. Options for the delayed phase include prophylaxis with a 5-HT3 antagonist, dexamethasone, or an NK1 antagonist plus dexamethasone.7,9,15
Patients receiving low emetogenic chemotherapy can be given a single dose of dexamethasone, a dopamine antagonist, or a 5-HT3 antagonist. Patients who are receiving minimally emetogenic chemotherapy generally do not require any preventive antiemetic.
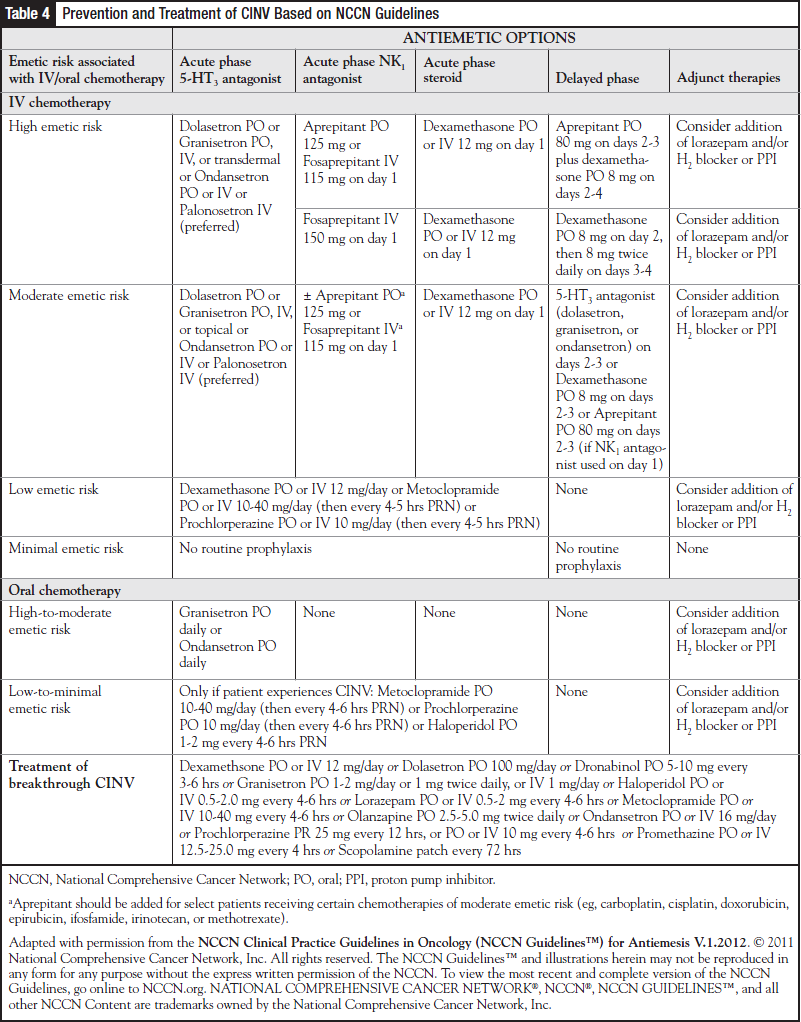
Patients receiving oral chemotherapy associated with a high or moderate emetic potential should be given an antiemetic medication before each dose of the oral chemotherapy.7,9,15 Those receiving oral chemotherapy with a low to minimal emetic potential, as-needed antiemetics should be sufficient. Recommendations from the more frequently updated NCCN guidelines are shown in Table 4.
Many patients have breakthrough CINV symptoms. Patients receiving moderately to highly emetogenic chemotherapy should be sent home with 1 to 2 medications to use for breakthrough symptoms, if they occur. The best choice of antiemetic medications would include agents from a different class with a different mechanism of action than those of the antiemetics given for prophylaxis.
Patients should be encouraged to take their as-needed antiemetics at the beginning of a nausea episode, before the symptoms get worse. Patients should be made aware that many medications used for breakthrough CINV can cause more sedation than other agents. If refractory CINV symptoms continue, the additional agents should be administered on a scheduled basis, and the patient should be evaluated for the need for IV hydration or for electrolyte supplementation.
In addition, the antiemetic effectiveness should be evaluated before the next cycle of chemotherapy, and potential modifications should be considered. These may include scheduling active as-needed medications, upgrading the antiemetic regimen to a regimen with greater emetogenic level, or adding olanzapine or a cannabinoid medication.
Patients who develop anticipatory nausea and vomiting may receive lorazepam the night before and the morning of the chemotherapy regimen, before they arrive at the treatment center. They may also be offered behavioral interventions, such as relaxation techniques or hypnosis.
Conclusion
Because of the multiple neurotransmitters and organs involved in the emetic response, multiple antiemetics, with different mechanisms of action are needed to prevent and treat CINV. By matching the antiemetic regimen to the emetogenic level of the chemotherapy regimen, patients can be offered the optimal preventive regimens. Pharmacists are uniquely positioned to ensure that patients receive the appropriate CINV prevention and treatment, to manage adverse effects, and to improve patient outcomes.
References
- Panesar K. Strategies for managing chemotherapy-induced nausea and vomiting. US Pharmacist. 2011;36(oncology suppl):12-15.
- Trigg ME, Higa GM. Chemotherapy-induced nausea and vomiting: antiemetic trials that impacted clinical practice. J Oncol Pharm Practice. 2010;16:233-244. Epub 2010 Jan 19.
- Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol. 2011;22:30-38. Epub 2010 Oct 14.
- Kris MG. Why do we need another antiemetic? Just ask. J Clin Oncol. 2003;21:4077-4080. Epub 2003 Oct 14.
- Roscoe JA, Morrow GR, Hickok JT, et al. Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manage. 2000;20:113-121.
- Haiderali A, Menditto L, Good M, et al. Impact on daily functioning and indirect/ direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a US population. Support Care Cancer. 2011;19:843-851. Epub 2010 Jun 9.
- National Comprehensive Care Network. NCCN clinical practice guidelines in oncology. Antiemesis. V1.2012. July 20, 2011. www.nccn.org/professionals/physi cian_gls/pdf/antiemesis.pdf. Accessed November 14, 2011.
- Grunberg SM, Warr D, Gralla RJ, et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity—state of the art. Support Care Cancer. 2011;19(suppl 1):S43-S47. Epub 2010 Oct 24.
- Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189- 4198. Epub 2011 Sep 26.
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482-2494.
- Geling O, Eichler HG. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systemic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005;23:1289-1294.
- Huang JQ, Zheng GF, Deuson R, et al. Do 5-hydroxytryptamine3 receptor antagonists (5-HT3) improve the antiemetic effect of dexamethasone for preventing delayed chemotherapy-induced nausea and vomiting (CINV)? A meta-analysis of randomized controlled trials. J Clin Oncol. 2004;22(14 suppl):Abstract 6037.
- Hickok JT, Roscoe JA, Morrow GR, et al. 5-Hydroxytryptamine receptor antagonists versus prochlorperazine for control for delayed nausea caused by doxorubicin: a URCC CCOP randomized controlled trial. Lancet Oncol. 2005;6:765-772.
- Billio A, Morello E, Clarke MJ. Serotonin receptor antagonists for highly emetogenic chemotherapy in adults. Cochrane Database of Systemic Reviews 2010. Issue 1. Article No: CD006272. Published online January 20, 2010. http://summaries. cochrane.org/CD006272/serotonin-receptor-antagonists-to-prevent-nausea-andvomiting- after-chemotherapy. Accessed November 14, 2011.
- Multinational Association of Supportive Care in Cancer. MASCC/ESMO antiemetic guideline 2011. April 2011. http://data.memberclicks.com/site/mascc/MASCC_Guidelines_English_2011.pdf. Accessed November 14, 2011.
- Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for the prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomized, comparative phase III trial. Lancet Oncol. 2009;10:115-124. Epub 2009 Jan 8.
- Botrel TE, Clark OAC, Clark L, et al. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systemic review and meta-analysis. Support Care Cancer. 2011;19:823-832. Epub 2010 May 22.
- Mattiuzzi GN, Cortes JE, Blamble DA, et al. Daily palonosetron is superior to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting in patients with acute myelogenous leukemia. Cancer. 2010;116:5659-5666.
- Yu Z, Liu W, Wang L, et al. The efficacy and safety of palonosetron compared with granisetron in preventing highly emetogenic chemotherapy-induced vomiting in the Chinese cancer patients: a phase II, multicenter, randomized, double-blind, parallel, comparative clinical trial. Support Care Cancer. 2009;17:99-102. Epub 2008 Sep 30.
- Likun Z, Xiang J, Yi B, et al. A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist. 2011;16:207-216. Epub 2011 Jan 31.
- Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy and tolerability analysis. Ann Oncol. 2007;18:233- 240. Epub 2006 Nov 15.
- The Italian Group for Antiemetic Research. Double-blind, dose-finding study of four intravenous doses of dexamethasone in the prevention of cisplatin-induced acute emesis. J Clin Oncol. 1998;16:2937-2942.
- The Italian Group for Antiemetic Research. Randomized, double-blind, dosefinding study of dexamethasone in preventing acute emesis induced by anthracyclines, carboplatin, or cyclophosphamide. J Clin Oncol. 2004;22:725-729.
- Celio L, Frustaci S, Denaro A, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer. 2011;19:1217-1225. Epub 2010 Jun 25.
- 25. Aapro M, Fabi A, Nolè F, et al. Double-blind, randomized, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. 2010;21:1083-1088.
- Curran MP, Robinson DM. Aprepitant: a review of its use in the prevention of nausea and vomiting. Drugs. 2009;69:1853-1878.
- Aapro MS, Walko CM. Aprepitant: drug-drug interactions in perspective. Ann Oncol. 2010;21:2316-2323. Epub 2010 May 20.
- Lasseter KC, Gambale J, Jin B, et al. Tolerability of fosaprepitant and bioequivalency to aprepitant in healthy subjects. J Clin Pharmacol. 2007;47:834-840. Epub 2007 May 24.
- Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol—EASE. J Clin Oncol. 2011;29:1495-1501. Epub 2011 Mar 7.
- Tan L, Liu J, Liu X, et al. Clinical research of olanzapine for prevention of chemotherapy induced nausea and vomiting. J Exper Clin Can Res. 2009;28:131-137.
- Jackson WC, Taverneir L. Olanzapine for intractable nausea in palliative care patients. J Palliat Med. 2003;6:251-255.
- Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25:578-582.
- Cruz FM, Cubero DIG, Taranto P, et al. Gabapentin for the prevention of chemotherapy-induced nausea and vomiting: a pilot study. Support Care Cancer. Epub 2011 April 5.