There has recently been a paradigm shift in the treatment of patients with cancer. Traditionally, cancer chemotherapy has been given through the intravenous (IV) route. However, in the past 15 years, the number of available oral chemotherapeutic agents has more than doubled. In addition, approximately 30% to 35% of antineoplastics currently being developed are in an oral formulation.1 Oral formulations offer many advantages for patients, including convenience, potential for reduced side effects, and enhanced quality of life. Several studies have shown that between 63% and 89% of patients would prefer an oral therapy if efficacy were not compromised.2-4
As oral chemotherapy becomes more widely utilized, many oncology healthcare providers will be unprepared for the challenges associated with these treatments. The potential challenges include procurement (ie, drug acquisition, insurance, and reimbursement issues), pharmacologic interactions, and patient safety factors (eg, appropriate patient education and safe handling of chemotherapeutic agents).
In 2007, a study by Weingart and colleagues surveyed 54 National Cancer Institute (NCI)-designated cancer centers regarding their safety practices for oral chemotherapy and concluded that US cancer centers have no consensus on how to safely process oral chemotherapy agents.5 In addition, there are no standards for prescribing, educating, or assessing patient adherence.5 Two years later, the American Society of Clinical Oncology (ASCO) and the Oncology Nursing Society (ONS) published joint guidelines on safe administration of chemotherapies, including requirements for standardization of ordering, preparation, patient education, monitoring, and follow-up with oral chemotherapy treatment.6 To date, these guidelines are the single source of guidance for institutions wishing to improve care of patients receiving oral antineoplastic agents.
At St Luke’s Mountain States Tumor Institute (MSTI), a retrospective review from our 5 outpatient cancer clinics showed that less than 25% of patients prescribed oral chemotherapy had utilized St Luke’s hospital- based outpatient pharmacies for their prescribed oral anticancer therapies within a 6-month period. In addition, a fair number of patients utilized more than 2 pharmacies, because of difficulty in obtaining oral chemotherapeutic agents. In an effort to comply with published standards, increase patient safety and continuity of care, as well as expand clinical oncology phar - macist services, we established a pharmacist-managed, interdisciplinary oral chemotherapy program.
The goals of the program were to develop prospective medication order review for oral chemotherapy; providing medication reconciliation; improving continuity of care, patient counseling, and patient education; monitoring for tolerability; follow-up for adherence; and increasing reimbursement revenue in the onsite out - patient pharmacies. We wanted to improve the oral chemotherapy prescription process in our facilities through standardized safeguards used during administration of IV therapy.
This article describes a novel program at St Luke’s MSTI, an NCI community cancer center program serving a broad geographic area that includes most of southern Idaho, eastern Oregon, and northern Nevada. The article evaluates workload requirements and shows how the program self-sustains by retaining revenue within the specific health system.
Methods
As a result of the need for expanded pharmacy services by St Luke’s MSTI and the initiative of a residency project, the process of establishing a new pharmacy service was begun. The initiation of the project required evaluation of standard legal and operating requirements of any pharmacy. Requirements included a physical space with basic necessities and meeting basic pharmacy needs, such as specialty licenses and insurance contracts.
Although we were able to secure an office for an oncology pharmacist to staff the oral chemotherapy program, we decided to utilize our Boise hospital’s onsite outpatient pharmacy for the actual filling of the oral chemotherapy prescriptions. This allowed us to circumvent the need for acquiring additional licensure and insurance contracts because they were already held by a pharmacy within the institution.
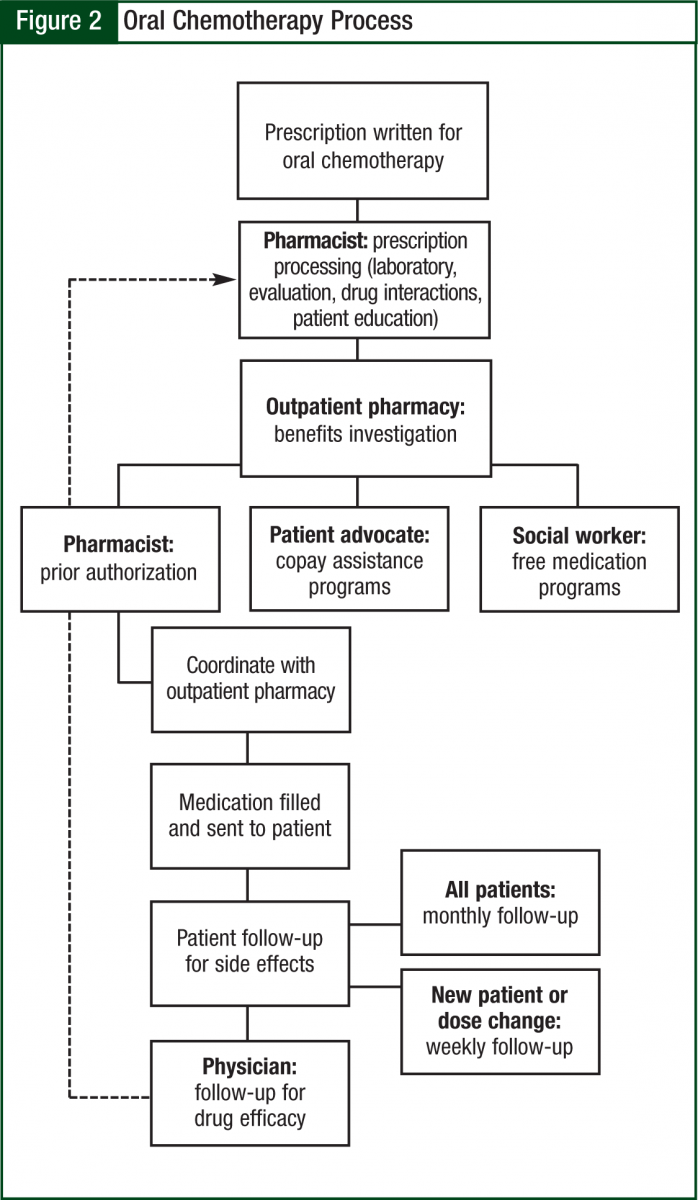
After securing the basic requirements, we determined a process of workflow based on the ASCO-ONS guidelines,6 taking into consideration the needs of our patient population and our particular institution. Close collaboration with multiple healthcare disciplines played an integral role in determining the actual process of oral chemotherapy processing. The groups included the outpatient (filling) pharmacy, clinic nurses, triage nurses, patient financial advocates, social workers, physician committees, and administrators. As a result of initial process complications discovered in the early phases of the program, we needed to solicit continual verbal feedback at monthly meetings from each of these groups in an ongoing process to improve our program and to best meet the needs of our patients and our facility.
To begin the process of filling oral chemotherapy prescriptions, we created 2 preprinted order forms, one for lenalidomide (Revlimid) and thalidomide (Thalomid) and the second for the remaining oral chemotherapeutic agents. Physicians prescribing oral chemotherapy were required to utilize the standardized forms, which include the following elements: the goal of treatment (eg, firstline, second-line, salvage), cycle length, cycle structure, whether the goal is prior authorization only or active dispensing, and a provision to allow the pharmacist to fill based on available pill sizes (Figure 1).
Initial contact with the patient was made within 24 hours to explain the filling process and to perform initial counseling on the medication. Once the patient had started the medication, the oncology pharmacist called the patient on a weekly basis for follow-up and assessment for the first cycle. Thereafter, the patient was called once per cycle before each refill to assess symptom management and adherence.
The oncology pharmacist kept in close communication with the prescribing oncologist and clinic nurses to ensure appropriate follow-up and assessment (including things such as laboratory work and symptom management). The average time to fill was 2 to 5 days, which is largely a function of insurance requirements. The pharmacy technician also schedules pick-ups with the patient, verifies receipt of mailed prescriptions, and assists in preparing the day’s schedule (Figure 2).
To justify this program, the oral chemotherapy office kept a daily log of workload that included the number of prescriptions filled and the number of follow-up phone calls. Interventions such as drug-drug interaction interventions, dose change suggestions based on laboratory results, and supportive care discussions were tracked indirectly. Revenue generated from oral chemotherapy prescriptions filled was tracked at the onsite outpatient pharmacy during fiscal year 2010 (October 2009-September 2010) and divided by month to determine the plateau of workload. In addition, the yearly costs and revenue of the program were tracked to ensure justification of the program and continued financial support of the required full-time equivalents. Other justification included surveying prescribing physicians and clinic nurses for satisfaction with the program. Finally, the program found a huge opportunity with lenalidomide counseling and distribution that is included.
Results
Workload Assessment
During a 16-month time period, the oral chemotherapy office had processed nearly 1500 prescriptions for 552 patients. One third of these prescriptions were new; the remaining two thirds were refills. The average workload was 30 prescriptions filled weekly, in addition to approximately 50 weekly follow-ups (via phone call or physician visit). All prior authorizations were completed by the oncology pharmacist and signed by the prescriber when necessary. Patients who were underinsured or uninsured were referred to our patient financial advocates to apply for copay assistance or free drugs from the manufacturer via that manufacturer’s drug assistance programs.
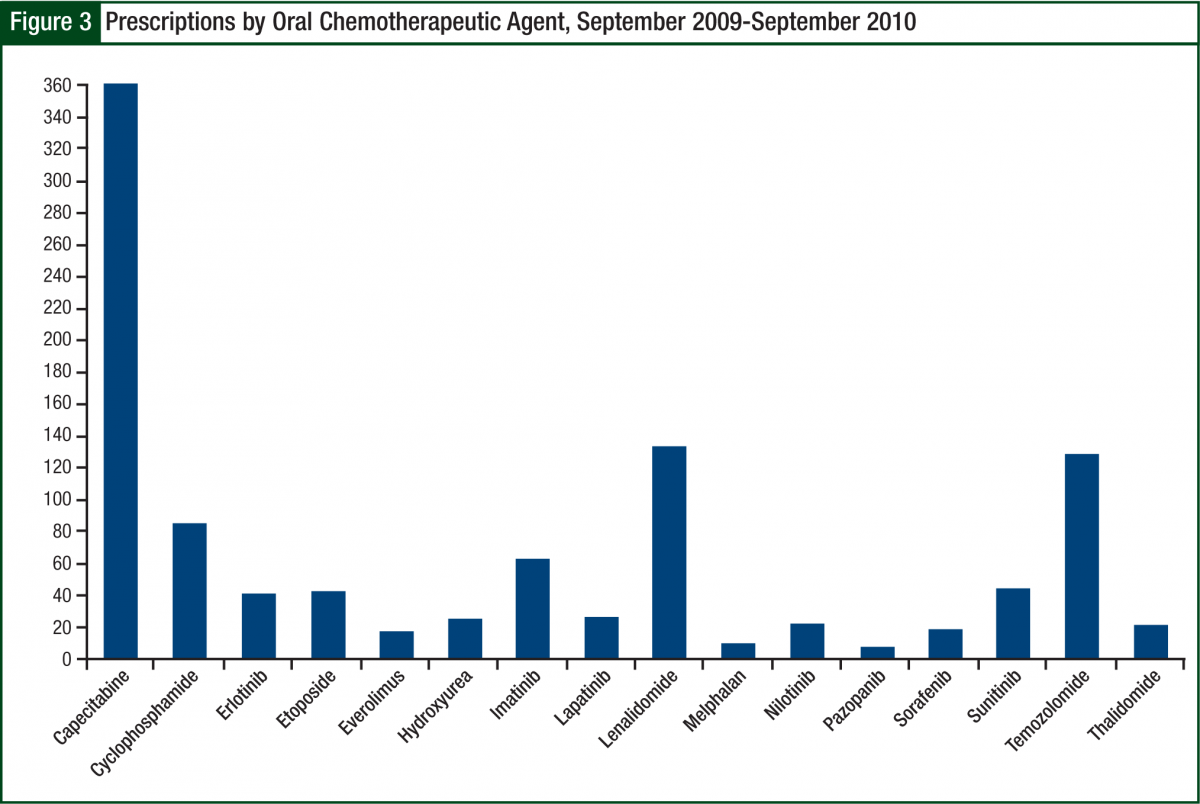
The most common prescription filled was capecitabine (Xeloda), followed by lenalidomide and temozolomide (Temodar) (Figure 3). Because of insurance requirements and contracts, approximately 10% of prescriptions referred to the oral chemotherapy office were required to go to a specific mail-order pharmacy. Before becoming certified to dispense lenalidomide, this number approached 20%, inferring that our current mail-order base is approximately 5% to 10%.
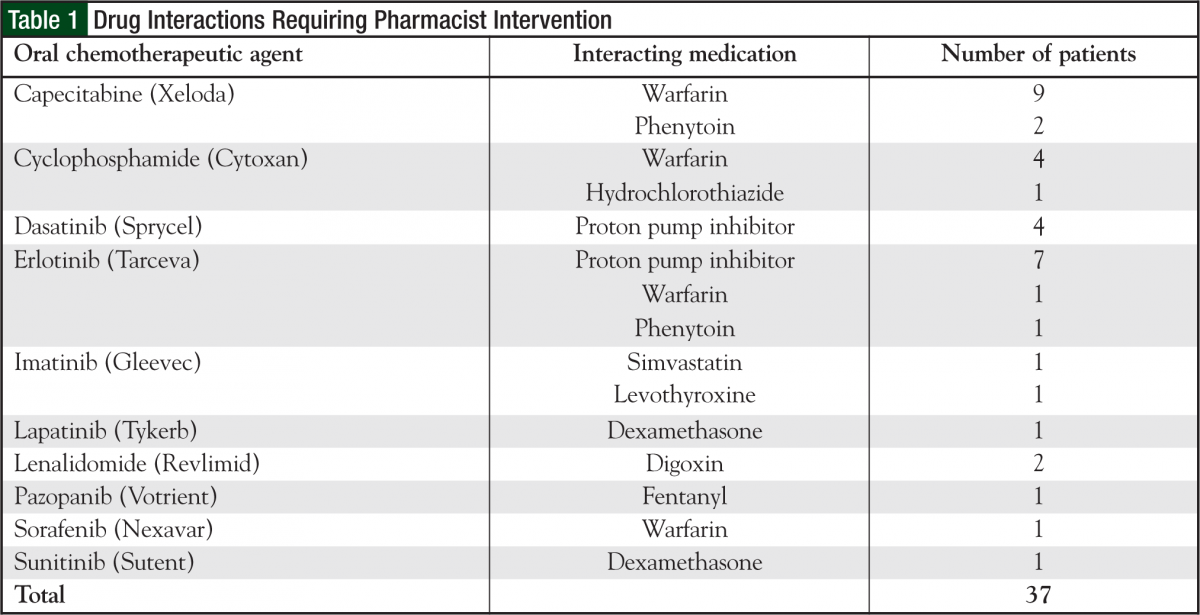
Although indirectly measured, the most common side effects discussed with patients included nausea/vomiting prophylaxis and treatment, management and prevention of hand-foot syndrome, diarrhea, stomatitis, and requirements for taking the medication (ie, with or without food). Of the 552 participating patients, 36 patients had a total of 37 drug interactions that required pharmacist intervention. The most common drug interactions found were with capecitabine, cyclophosphamide, and certain tyrosine kinase inhibitors, specifically erlotinib (Tarceva) and dasatinib (Sprycel) (Table 1). In addition, 11 patients required dose adjustments from the originally prescribed dose because of laboratory values (eg, serum creatinine, bilirubin). All dose adjustment recommendations were accepted by the physicians.
Revenue and Program Sustainment
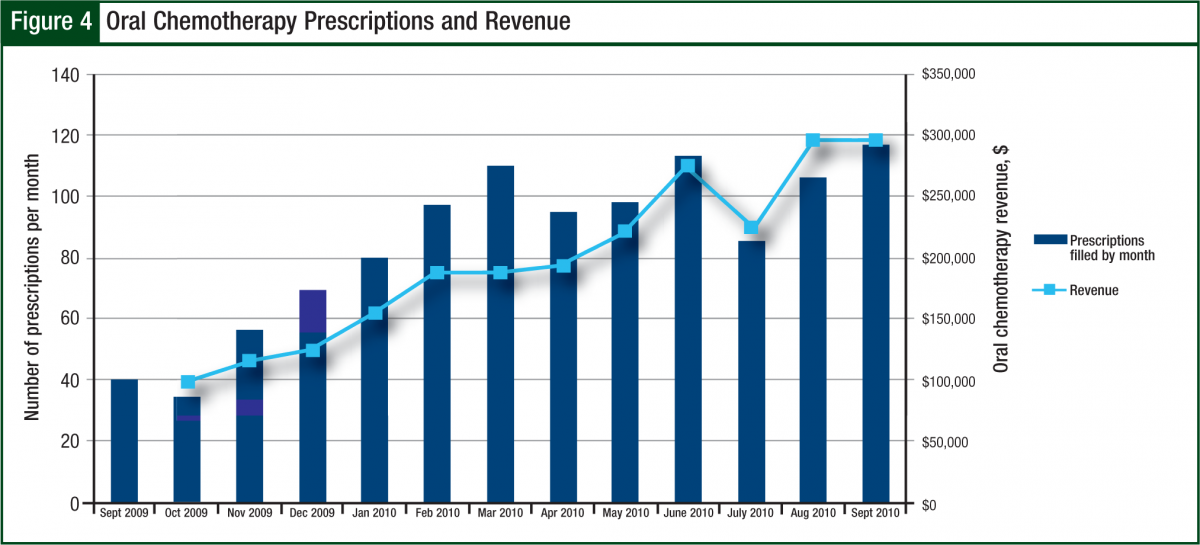
During fiscal year 2010, the number of prescriptions had an average 3-fold increase from 40 new and refill prescriptions per month in October 2009 to 120 per month by September 2010. The revenue generated matched this trend by increasing from $100,000 per month to $300,000 per month by the end of the fiscal year (Figure 4).
Based on current monthly estimates, this program will result in yearly gross revenue of $2.4 million, double the original estimate of $1.2 million. The total annual operating costs for the program were approximately $230,000, including salaries, overhead, educational brochures, and mailings. Based on typical profit margins from the gross revenue, this program more than pays for itself. In addition, with the increased revenue from the program, several patients were able to get free drugs for their first cycle, while assistance paperwork was pending, to ensure timely initiation of therapy within a couple days as opposed to weeks or more until insurance issues were resolved.
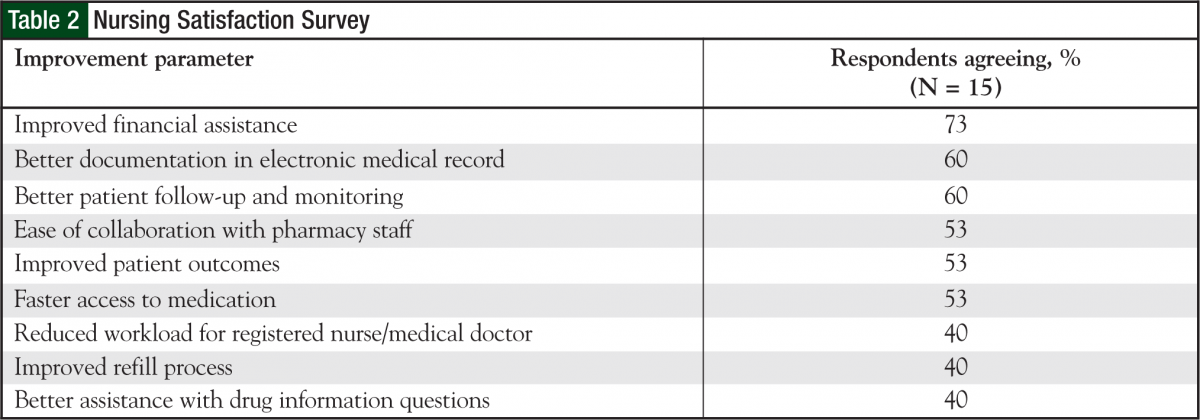
A total of 15 oncology nurses, of the 25 who were approached, responded to a satisfaction survey initiated by the outpatient chemotherapy program pharmacists. Of the 15, 83% were very satisfied or satisfied with the process overall. Also, 80% were at least satisfied with the refill process, and 86% were at least satisfied with the copay assistance work integrated between the financial advocates and the oral chemotherapy office. According to the nurses, most patients spoke highly about the ease of obtaining/picking up medications and refills, copay assistance, and follow-up phone calls. All the nurses commented that it was moderately to very easy to contact the pharmacist working in the oral chemotherapy office. Most nurses had very positive interactions with the oral chemotherapy pharmacists (Table 2).
Some of the positive comments from the nurses were, “This process is a HUGE improvement over what we were doing; patients LOVE it.” And, “The benefit of this program is that it takes the detailed work and attention given to IV chemotherapy for years and finally applies it to oral chemotherapy. It’s all about patient safety and teaching.”
With regard to future improvements, nurses requested the pharmacy provide follow-up for patients utilizing mail-order pharmacy, as well as for those whose prescriptions are filled at the onsite outpatient pharmacy.
A total of 7 of our 10 medical oncologists at 5 sites responded to our physician-prescriber satisfaction survey. Of these 7 respondents, 5 were extremely satisfied with the way the program is operating, all of whom believed this program has provided better access and thus better outcomes for their patients. Two physicians were indifferent, because they personally did not see a change in their workload but felt that the best response to the survey questions should be ascertained from their patients. The physicians also recommended some changes in how the pharmacists document fill dates, interventions, and follow-ups so that they can more easily determine when the patients start their cycles and/or pick up their medications. Lenalidomide and RevAssist REMS program Lenalidomide is more time-intensive and challenging to fill compared with other oral chemotherapeutics, because of the risk evaluation and mitigation strategy (REMS) restrictions.7 For a pharmacy to fill lenalidomide, the pharmacy must complete a training program for certification of educators, which can be either nurses or pharmacists. Each month before the fill, the certified counselors call the patient to review a standardized counseling checklist.
The counselors are also responsible for reporting any adverse reaction(s) the patient experiences while receiving this medication. The pharmacy is responsible for keeping a recorded inventory that includes the strength, lot number, and quantity of lenalidomide dispensed to the patient. Because of the workload required, it is essential to assess whether it is feasible for the health system to take on this responsibility.
After the first 6 months of evaluating the program, a significant potential loss to follow-up in patients taking lenalidomide was discovered. Therefore, a decision was made to enroll the oral oncology pharmacists as certified RevAssist counselors so that the institution could maintain these patients internally. This allowed the second most common prescription to be brought back within the health system, further enhancing care for patients and retaining revenue for the institution. In addition, St Luke’s MSTI was able to justify a half-time technician position and a pharmacy biller to assist the oncology pharmacist.
Conclusion
Since the US Food and Drug Administration approval of capecitabine in 1998, there has been a shifting paradigm in cancer care toward oral chemotherapeutics.8 The implications for this are huge—not just for patients, but for cancer centers as well. When prescriptions are sent to an external pharmacy, there is no income provided to the cancer center. This is potentially problematic, because staff time involved in assisting patients with obtaining their medications is not billable.9
St Luke’s MSTI’s oral chemotherapy program has allowed for revenue to stay within the health system each year to continually fund this program. Previously, only 25% of patients were filling their prescriptions at our health system pharmacies. Based on data generated from the electronic medical records, an estimated 75% to 85% of prescriptions now remain within our health system, thus allowing consistent follow-up our patients previously did not receive. This change has the potential to improve compliance and accessibility and decrease patient concerns.10
A unique advantage to keeping these prescriptions within our health system is that drug interactions, dose adjustments, and supportive care referrals can be done in a more timely fashion, thereby preventing further delays in initiation of treatment. In addition, consistent documentation and follow-up within one system allows for improved continuity of care for patients and for enhanced safety outcomes.6
This program could not have been a success without the strong collaboration among individuals with varying areas of specialization, including the oncologists, nurses, pharmacists, retail pharmacy team, billing specialists, patient financial advocates, and social workers, as well as the insight of our pharmacy business analyst. Future directions of this program involve continued adaptation of new oral oncologic agents, as well as integration of the supportive care medications prescribed in the oncology population, such as deferasirox (Exjade) or eltrombopag (Promacta). An additional challenge is to find a way to include follow-up for the 5% to 10% of patients who receive oral chemotherapy from mail-order pharmacies.
With our current workload, patients who utilize mailorder pharmacies are difficult to include, but future changes may allow us to integrate them into our system to improve monitoring and follow-up with these patients. In addition, examination of the impact of this program, including adherence rates, average duration of time on oral chemotherapeutics, and supportive care management and counseling, are also needed. Because one third of our fills are new prescriptions, we believe that many patients do not continue taking oral chemotherapeutics as long as may be predicted and often stop after a few cycles as a result of toxicity and/or disease progression.
The ultimate finding is that oncology pharmacists have a unique opportunity to manage patients’ prescribed oral chemotherapy. This includes the ability to help the health system comply with REMS programs, as seen with lenalidomide, and improve safe-handling practices by helping establish procedures for ordering, filling, and dispensing oral chemotherapy. The program described here has become an integral collaborative practice, as well as a self-sustainable program for our not-for-profit health system.
Author Disclosure Statement
Drs Mancini, Kaster, Vu, Modlin, and Mr Wilson have reported no actual or potential conflicts of interest.
References
- DeCardenas R, Helfrich J. Oral therapies and safety issues for oncology practices. Oncology Issues. 2010;(March/April):40-42.
- Fallowfield L, Atkins L, Catt S, et al. Patients’ preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancer. Ann Oncol. 2006;17:205-210.
- Borner M, Schöffski P, de Wit R, et al. A randomized crossover trial comparing oral UFT (uracil/tegafur) + leucovorin (LV) and intravenous fluorouracil (FU) + LV for patient preference and pharmacokinetics in advanced colorectal cancer. Proc Am Soc Clin Oncol. 2000;19:Abstract 741.
- Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110-115.
- Weingart SN, Flug J, Brouillard D, et al. Oral chemotherapy safety practices at US cancer centres: questionnaire survey. BMJ. 2007;334:407.
- Jacobsen JO, Polovich M, McNiff KK, et al. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. Oncol Nurs Forum. 2009;36:651-658.
- Revlimid (lenalidomide) [package insert]. Celgene Corporation: Summit, NJ; January 2009.
- Choi S, Boehnke L. Oral chemotherapy: a shifting paradigm affecting patient safety. Hem Onc Today. November 25, 2008. www.hemonctoday.com/article.aspx?rid=33070. Accessed April 28, 2011.
- Hede K. Increase in oral cancer drugs raises thorny issues for oncology practices. J Natl Cancer Inst. 2009;101:1534-1536.
- Increased use of oral chemotherapy drugs spurs increased attention to patient compliance. J Oncol Pract. 2008;4:175-177.