Background: Cancer of the testis consists of a diverse group of neoplasms, most of which are germ-cell tumors. Although testicular cancer comprises only 2% of all malignancies in the United States, it is the most common solid tumor in young men between the ages of 15 and 34 years. Advances in the management of testicular germ-cell tumors over the past 40 years have resulted in great improvement in the management of this disease.
Objective: To review the historical and clinical significance of chemotherapy in the management of testicular germ-cell tumors.
Discussion: The treatment of testicular germ-cell tumors involves 4 modalities, including surgery, radiation, chemotherapy, and observation. All patients with a testicular primary tumor will undergo a radical inguinal orchiectomy. The timing of the surgery is typically conducted before chemotherapy or radiation; however, some cases may warrant a delay in surgery to proceed with chemotherapy first, in which case the diagnosis is made clinically based on elevated tumor markers. Current advances in the management of patients with testicular cancer have resulted with a change from a disease with 90% mortality to a disease with almost a 90% cure rate with first-line chemotherapy. Long-term survival can also be achieved with second- and third-line chemotherapy regimens.
Conclusion: With overall cure rates of more than 90%, this disease is the model of care for curable cancer. Patients with refractory or with relapsed disease can still be cured with second- or third-line chemotherapies with cisplatin-based regimens or with high-dose chemotherapy and transplantation. Pharmacists caring for this patient population must monitor for common toxicities associated with chemotherapy regimens.
In 2011 it was estimated that 8290 new cases of testicular cancer would be diagnosed in the United States.1 Cancer of the testis consists of a morphologically and clinically diverse group of neoplasms, most of which are germ-cell tumors. Although testicular cancer comprises only 2% of all malignancies in the United States, it is the most common solid tumor in men between the ages of 15 and 34 years.2
The incidence of testicular germ-cell tumors is highest in men of Scandinavian, German, and New Zealand descent, and it is principally observed in whites. The cause of testicular germ-cell tumors is unknown; nevertheless, the risk factors are known and include family history, cryptorchidism, Klinefelter’s syndrome, and testicular dysgenesis.3
Initial Presentation and Management
The characteristic presentation of testicular germ-cell tumors is a painless testicular mass; however, most patients will present with symptoms of epididymitis and/or orchitis, which are painful and uncomfortable. A trial of antibiotics is usually prescribed to treat presumptive epididymitis, and if no response is observed, a testicular ultrasound is required. On ultrasound, the typical tumor is intratesticular and produces a hypoechoic mass. A radical inguinal orchiectomy with ligation of the spermatic cord is required for all patients with suspected testicular cancer. Transscrotal biopsy with orchiectomy is contraindicated, because of the potential to introduce metastatic spread. In addition, computed tomographic imaging of the chest, abdomen, and pelvis are required for staging of disease.3
Primary extragonadal disease comprises 10% of all testicular germ-cell tumors, which occurs most frequently in the mediastinum and retroperitoneum. Patients with mediastinal involvement may present with shortness of breath and chest pain, and potentially with superior vena cava syndrome. Patients with retroperitoneum involvement present with back pain or with an abdominal mass. Regardless of the presentation, a testicular ultrasound is required to diagnose the primary site of malignancy.3
Testicular germ-cell tumors are histologically classified as seminomatous or nonseminomatous.4 Approximately 50% of men present with seminoma histology and 50% present with nonseminomatous features. Nonseminomatous tumors are divided into 4 subclassifications, including choriocarcinoma, embryonal carcinoma, teratoma, and yolk sac tumor. Nonseminomas are more aggressive than seminomas, and when both are present, the management of nonseminoma takes precedence. Pathologic specimens often contain several mixed histologic entities.4 Common sites of metastases include the retroperitoneum, retroperitoneal lymph nodes, lungs, and brain.3
Serum tumor markers are used for diagnosis, staging, and assessing response to different modalities of treatment. These tumor markers are beta-human chorionic gonadotropin (b-hCG; normal range, <3 mIU/mL), alpha-fetoprotein (AFP; normal range, 0-25 ng/mL), and lactate dehydrogenase (LDH). AFP production is restricted to nonseminomatous germ-cell tumors, specifically embryonal carcinoma and yolk sac tumor. Elevations in AFP may also occur during liver damage (infectious or drug- or alcohol-induced), hepatocellular carcinoma, and other gastrointestinal tract cancers. Elevated b-hCG may be observed in seminomatous and in nonseminomatous tumors; false-positive b-hCG results include cross-reactivity of the antibody with luteinizing hormone, hypogonadism, and marijuana use. The serum half-lives of b-hCG and AFP are 1 and 5 days, respectively. Increased levels of b-hCG occur in 40% to 60% of patients with metastatic nonseminomatous germ-cell tumors, and in 15% to 20% of patients with metastatic seminomas. LDH is less specific, but concentrations are increased in 60% of patients with nonseminomatous testicular cancer and in 80% of those with seminomatous testicular cancer.3
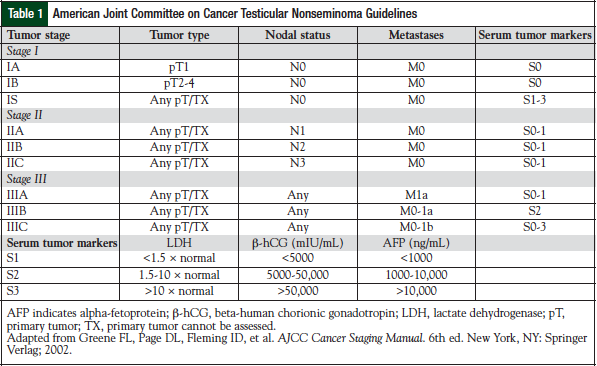
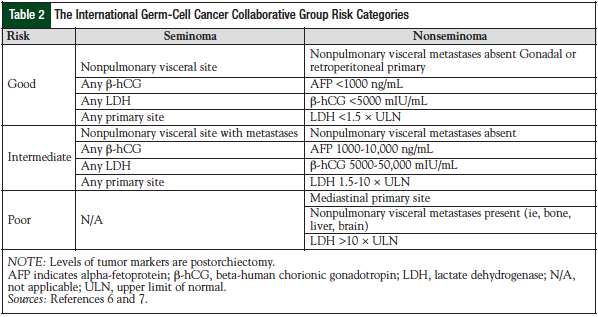
Testicular germ-cell tumors are treated based on the stage of disease and the risk factor classification.5,6 Stage I disease is confined to the testis; stage II disease includes spreading to the retroperitoneal lymph nodes; stage III is supradiaphragmatic disease, with nodal metastases to the posterior mediastinum or with supraclavicular region or hematogenous spread to nonnodal sites, particularly to the lungs. The American Joint Committee on Cancer has issued standard guidelines for testicular cancer staging (Table 1).5 The International Germ Cell Cancer Collaborative Group (IGCCCG) risk status criteria should be used in all clinical trials and in treatment decisions for patients requiring chemotherapy for advanced disease (Table 2).6,7
Evolution of Testis Cancer Treatment
as a Clinical Model
Although testicular germ-cell tumor only accounts for 2% of all malignancies, it is an important disease in oncology. It has been a model for multidisciplinary care and the systematic approach to clinical trial evaluation of chemotherapy agents in cancer (Figure 1).7-25 It has also been a testing ground for new antineoplastic drugs. The US Food and Drug Administration primarily approved cisplatin, ifosfamide, and etoposide based on evidence in testicular germ-cell tumors. These 3 chemotherapeutic agents are now common treatments for patients with many types of cancer, such as small-cell lung cancer, non–small-cell lung cancer, relapsed lymphoma, sarcoma, and head and neck cancers. The treatment of testis cancer represents a landmark achievement in cancer drug development, demonstrating dramatic improvements in cure rates with the use of multiagent chemotherapy.
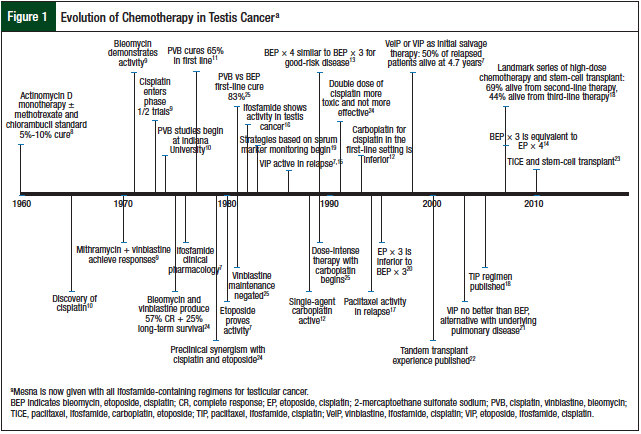
Research into the efficacy of combination chemotherapy for testicular germ-cell tumors began almost 40 years ago (Figure 1). The hypothesis was that malignant cells would rapidly develop drug resistance to single-agent therapy, leading to disease progression and subsequent death. The goal of combination chemotherapy was to (1) use drugs with single-agent activity based on different mechanisms of action, (2) use drugs with nonoverlapping toxicity, and (3) whenever possible, combine therapies with synergistic killing rather than with just additive effects.
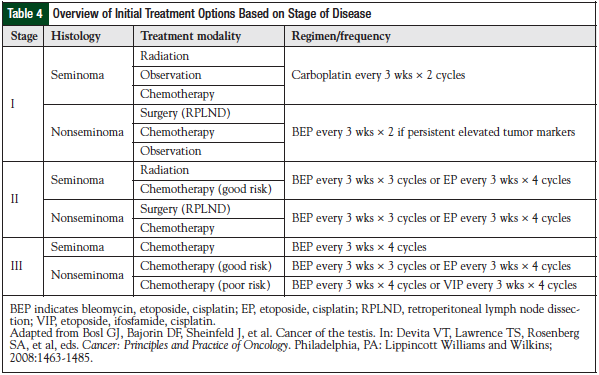
Initial therapy with actinomycin D produced 5% to 10% cure rates in the early 1960s.8 The discovery of vinblastine plus bleomycin as first-line therapy resulted in 25% long-term disease-free survival.9 The opportune discovery of cisplatin (formerly cis-diamminedichloroplatinum) and its incorporation into the management of patients who progressed from therapy with actinomycin D produced complete responses, with durable remissions.26 This led to the systematic incorporation of cisplatin with vinblastine and bleomycin (PVB), which produced complete remissions in 74% of patients with disseminated disease after first-line chemotherapy, and an additional 11% complete remissions after post-PVB surgical resection.10 Overall, this approach provided long-term disease-free survival of 64% at 30 months.10
All subsequent studies addressed clinically relevant questions, such as:
• What is the number of cycles required for initial therapy?
• Is maintenance therapy required?
• Can cisplatin be substituted for carboplatin?
The dogma of maintenance therapy with vinblastine was challenged and proved unnecessary after first-line treatment. As novel agents were discovered (most recently, etoposide) and were shown to be active in the preclinical arena, they were tested in patients with multiple relapsed disease, then as initial salvage therapy, and finally as frontline therapy. The standard PVB regimen was then compared head to head with bleomycin, etoposide, and cisplatin (BEP), producing disease-free results in 74% and 83% of patients, respectively, with a noted survival advantage for advanced disseminated disease.11
Carboplatin was also investigated as a substitute for cisplatin in combination with bleomycin and etoposide for the treatment of patients with good-risk, metastatic, nonseminomatous disease.12 Chemotherapy consisted of 4 cycles of carboplatin with an area under the curve of 5 mg/mL/min, etoposide, and bleomycin (CEB) versus BEP. Complete responses were more common in patients receiving BEP (94.4%) compared with CEB (87.3%; P = .009), indicating that carboplatin should not be used as a standard of care.12 The primary end point of failure-free survival at 1 year was inferior for the carboplatin arm at 77% versus BEP at 91% (P <.001).12
Next, the number of cycles of BEP was evaluated in patients with good-risk disease in an effort to minimize the total number of cycles required for optimal treatment. The Southeastern Cancer Study Group trial demonstrated that 3 versus 4 cycles of BEP did not show a significant difference (P = .8) in overall survival (OS) or in disease-free survival (P = .93), with a median follow-up of 10.1 years.13 This changed the standard of care to consist of only 3 cycles of BEP in patients with good-risk disease.13 From here, salvage treatment continued to evolve to include novel agents and the incorporation of high-dose chemotherapy followed by stem-cell transplantation.
Selection of Initial Treatment
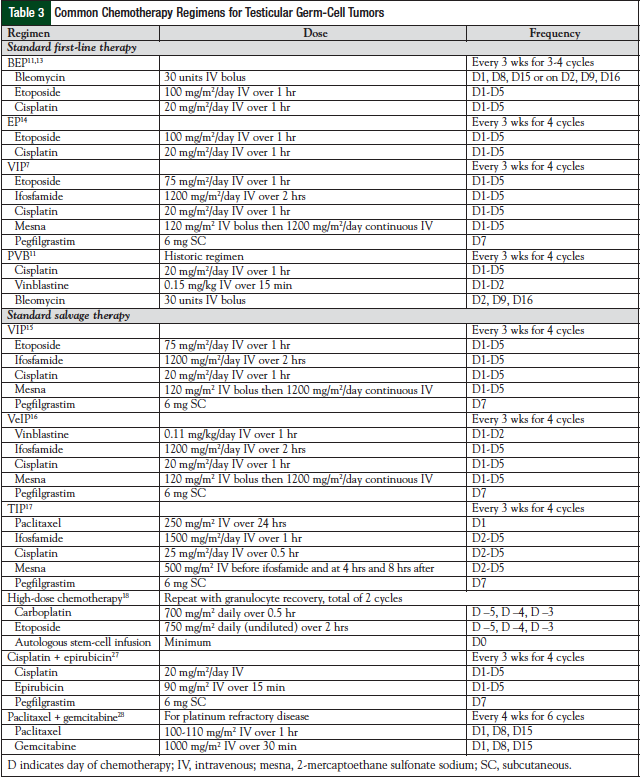
The treatment of testicular germ-cell tumors involves 4 different modalities—surgery, radiation, chemotherapy (Table 3),7,11,13-18,27,28 and observation. All patients with a testicular primary tumor will undergo a radical inguinal orchiectomy. The timing of the surgery is typically conducted before chemotherapy or radiation; however, some cases may warrant a delay in surgery to proceed with chemotherapy first (ie, when a patient is hemodynamically unstable). In that case, the diagnosis is made clinically based on elevated tumor markers. If orchiectomy is delayed, the procedure should be completed soon after chemotherapy, because the testes serve as a sanctuary site, and the ability to penetrate the testes with chemotherapy is extremely poor.
Stage I Seminoma
In stage I seminoma, cancer is confined to the testes. After completing orchiectomy, treatment will depend on the histology (ie, seminoma or nonseminoma). Therapies to treat stage I seminoma include radiation, observation, or chemotherapy. Seminomas are exquisitely responsive to radiation therapy. Typical progression and spread of disease occur through the retroperitoneal lymph nodes; therefore, radiation is directed to the retroperitoneum, including the para-aortic lymph nodes and potentially the ipsilateral iliac nodes.29
Observation is also an acceptable management option in patients with stage I seminoma. In approximately 15% to 20% of patients, the disease will relapse while undergoing surveillance.3 Most relapses occur 12 to 15 months later (which is later than with nonseminoma).3 In a 15-year review of patients with stage I seminoma treated with adjuvant radiotherapy compared with patients with active surveillance, the 5-year relapse-free survival and OS were 95% and 100%, respectively.30 Some risk factors that have been hypothesized to increase the risk of relapse include primary tumor size >4 cm and rete testis invasion, but no randomized trials have prospectively evaluated these risks. Surveillance allows the avoidance of unnecessary treatment in the majority of patients whose disease would likely never recur; this will also avoid exposing them to toxicities of chemotherapy.
Chemotherapy has been evaluated in stage I seminoma using single-agent carboplatin. Patients were randomized to radiation therapy to the para-aortic lymph nodes or to 1 cycle of carboplatin.31 After a median follow-up of 6.5 years, relapse-free survival rates were similar across groups (96% with radiation vs 94.7% with single-agent carboplatin; hazard ratio, 1.25; 90% confidence interval, 0.83-1.89). Toxicities were very similar across the groups. Based on these data, carboplatin has been added to the standard treatment guidelines for stage I seminoma.31
Stage I Nonseminoma
Radiation therapy does not play a role in the treatment of patients with stage I nonseminoma testicular cancer. Treatment modalities include observation, bilateral retroperitoneal lymph node dissection (RPLND), or chemotherapy. Approximately 25% to 30% of patients will relapse from their disease, and few patients will relapse after 2 years.19 Observation can be used to avoid the morbidity associated with RPLND surgery, with the understanding that cisplatin-based chemotherapy can cure recurrent disease. When using observation for patients with nonseminomatous testicular cancer, it is likely that these patients will require both RPLND and chemotherapy at disease relapse. The retroperitoneum is the most common site of relapse in patients.19 Other common sites of recurrence include the lungs, brain, or other visceral sites. Because the retroperitoneum is a common site of relapsed disease, RPLND plays an invaluable role in the treatment of this disease.19 Observation should only be offered to a very compliant patient, because it is imperative to undergo routine, close monitoring.3
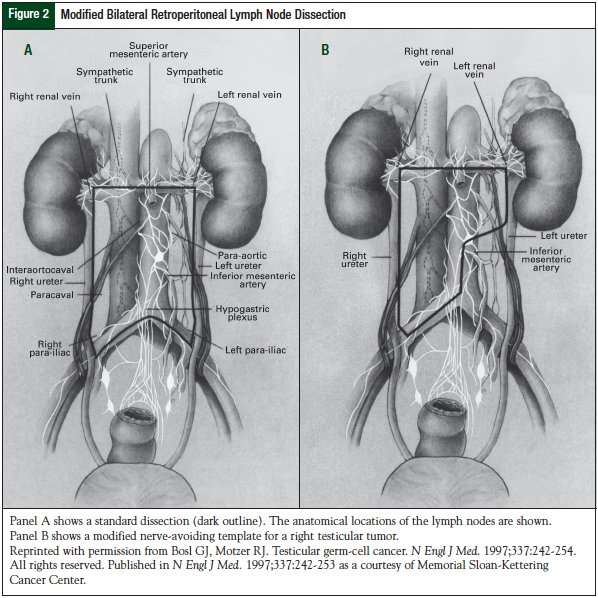
RPLND serves as a diagnostic procedure to define the presence or absence of disease, as well as to remove the most common area to find relapsed disease (Figure 2).19 This surgical procedure is associated with <1% mortality, and rare complications such as hemorrhage, bowel obstruction, and pulmonary embolism have been reported.32 The most common long-term morbidities associated with RPLND are retrograde ejaculation, infertility, and urinary and fecal incontinence resulting from nerve damage from the surgery. It is crucial to have an experienced surgeon for this surgery, who also uses nerve-sparing techniques to reduce long-term morbidity and ensures complete resection of the disease. With the use of nerve-sparing surgery, >95% of patients have preservation of antegrade ejaculation.32
Chemotherapy has been evaluated in patients with stage I nonseminoma. In the trial by de Wit and Fizazi, BEP for 2 cycles resulted in a 5% relapse rate and 1% death rate from disease after receiving chemotherapy.33 The German Testicular Cancer Study Group randomized 382 patients with stage I nonseminoma testicular cancer to 1 cycle of BEP or RPLND after orchiectomy.34 Because of toxicities associated with chemotherapy, postorchiectomy chemotherapy is not routinely used. There is, however, a group of patients in whom chemotherapy would be warranted, such as those with persistent elevated serum tumor markers after surgery but with no radiologic finding of disease.35,36 Chemotherapy in this group of patients would consist of 4 cycles of etoposide and cisplatin (EP) or 3 cycles of BEP.
Stage II and III Seminoma
In patients with stage II seminoma (stages IIA or IIB), the treatment of choice is radiation therapy.3 The radiation fields are similar to those for stage I treatment, but the fields are widened to include para-aortic or pelvic adenopathy. In patients with stage IIC good-risk disease, chemotherapy consisting of either 4 cycles of EP or 3 cycles of BEP is recommended.13,20 In seminoma, all stage III disease is considered good-risk disease, except in patients who have nonpulmonary visceral metastasis. Patients with stage III intermediate-risk seminoma who have nonpulmonary visceral metastasis should be given chemotherapy with 4 cycles of BEP. If relapse occurs, second-line chemotherapy is administered with a cure rate of >90% with advanced seminoma.19
Stage II and III Nonseminoma
In stage II nonseminoma, if patients are experiencing persistent elevations of their tumor markers after orchiectomy, treatment consists of RPLND and/or chemotherapy.3 After surgery, more than 65% of patients will be cured of disease and not need chemotherapy.19 In patients with normal tumor markers, an RPLND alone is the standard of care.3 Close follow-up is important in these patients, and the decision to use adjuvant chemotherapy versus observation is based on the ability of the patient to adhere to close follow-up. Many patients will opt for adjuvant chemotherapy consisting of the standard BEP or EP regimens (Table 2). If the disease relapses during the observation period, chemotherapy with BEP or EP is administered. Clinical trial results have demonstrated equivalence between 4 cycles of EP and 3 cycles of BEP, and the hematologic and nonhematologic toxicities were similar.20 After a 10-year follow-up, no significant difference was observed in OS and disease-free survival (P = .80 and P = .93, respectively) between the treatment arms.13
Stage III nonseminomas are classified into good-, intermediate-, and poor-risk diseases. Although stage III is considered to be a metastatic disease, cisplatin-based chemotherapy will cure approximately 75% of patients with stage III disease.11 The standard chemotherapy for patients with good-risk stage III disease would consist of 3 cycles of BEP, with which approximately 90% of patients are cured.13
Treatment for intermediate-risk, nonseminomatous, metastatic testicular cancer has been evaluated in a randomized clinical trial consisting of 4 cycles of BEP versus 4 cycles of etoposide, ifosfamide, and cisplatin (VIP) in 84 patients.21 Complete response rates were similar between the treatment arms (79% vs 74%; P = .62, respectively), and after a follow-up of more than 7 years, relapse-free survival, disease-free survival, and OS rates were similar. The ifosfamide-containing arm did produce significantly more myelosuppression compared with the BEP arm. Based on toxicities and no overall efficacy advantage, 4 cycles of BEP are considered the standard of care for patients with intermediate-risk nonseminoma.
In poor-risk disease, patients will receive chemotherapy consisting of 4 cycles of BEP or 4 cycles of VIP (Table 3). The VIP regimen would be chosen over BEP in patients with extensive lung disease. The use of bleomycin is avoided, because of the known pulmonary fibrosis that can occur.3 Also, worsening lung function could exclude patients from undergoing postchemotherapy resection of their disease. If patients have preexisting pulmonary dysfunction, they should not receive bleomycin. The 7-year OS rate (VIP, 69% vs BEP, 67%) and the progression-free survival (PFS) rate (VIP, 64% vs BEP, 58%) are considered equivalent between the 2 groups, with VIP an alternative treatment option (Table 4).3
Posttreatment Follow-up
After chemotherapy has been completed, patients follow up with radiologic tests and serum tumor markers to evaluate for residual disease. In the event of residual disease, it is imperative to remove it surgically. As many as 45% of the resected tumors will contain necrotic tissue, approximately 42% will contain teratoma (which can eventually transform to other malignancies, such as sarcoma), and approximately 10% of tissue will have viable tumor remaining (which is likely resistant to chemotherapy).37 If the surgical resection reveals residual disease, 2 additional cycles of chemotherapy can be considered.37 In patients with residual disease at sites other than the retroperitoneum, surgical resection is also extremely important, because there is a higher likelihood of teratoma outside of the retroperitoneum. This is especially true in patients with mediastinal testicular cancer, in whom any mediastinal mass would need to be surgically resected.
The expectation is that after therapy the serum tumor markers will return to normal levels. However, there is a subgroup of patients in whom, despite appropriate therapy with orchiectomy and chemotherapy, tumor markers do not decline. In the past, chemotherapy had been traditionally offered to these patients. Two retrospective trials have been conducted in this patient population (ie, those with metastatic testicular cancer) to identify the role of surgery.38,39 In both trials, the 5-year OS rate was approximately 54% to 57% of patients who underwent surgery.38,39 The most important factor of successful treatment was complete resection of the disease. Factors identified to be poor predictors of response included increased βb-hCG levels, serum AFP levels, redo RPLND, and testicular germ-cell cancer in the resected specimen.38,39 In select patients, surgical resection after persistent elevated serum tumor markers can achieve long-term success (ie, disease-free survival) and should be conducted by an experienced surgical oncologist.
Relapsed Disease
In up to 35% of patients with testicular germ-cell tumors, the disease will relapse after standard treatment.19 These patients are candidates for salvage therapy, and the goal is still cure. The concept for salvage therapy has been to use cisplatin plus other active agents that were not initially used in first-line treatment. In 1978 etoposide was combined with cisplatin as salvage therapy for advanced, refractory testicular germ-cell tumors.40 Among 33 patients, 14 complete remissions and 15 partial remissions were observed, with dose-limiting toxicities being myelosuppression.40 Based on this experience, the Southeastern Cancer Study Group initiated a trial of etoposide and cisplatin, sometimes in conjunction with bleomycin and/or doxorubicin, for the treatment of refractory testicular germ-cell tumors.41 Of the 44 patients enrolled, 18 received cisplatin plus etoposide, and another 18 received cisplatin plus etoposide and bleomycin. The remainder included doxorubicin therapy. Of the total patients, 44% achieved a complete remission and 27% achieved a partial remission. The actuarial 5-year survival was 28%, with patients who achieved a complete response expected to have a 72% survival.41 This study proved that sustained disease-free survival after second-line therapy in relapsed metastatic disease is a realistic treatment goal.
Preclinical studies showed promise with ifosfamide as a single agent in refractory testicular germ-cell tumors; however, the initial clinical utility of drug administration was complicated by the high incidence of hemorrhagic cystitis requiring an antidote, 2-mercaptoethane sulfonate sodium (mesna).42 Now mesna is given with all ifosfamide-containing regimens for testicular cancer.42
VIP and VeIP
Given the clinical activity with other standard chemotherapeutic agents, the use of VIP or vinblastine, ifosfamide, and cisplatin (VeIP) was studied in patients with recurrent disease who received 2 previous cisplatin-containing regimens.43 Of the 56 evaluable patients, 21% attained a complete remission with a median response duration of 34 months.43 The addition of ifosfamide to the cisplatin-based chemotherapy provided a plausible option for achieving a sustained remission with third-line therapy. Accordingly, the VeIP regimen was evaluated as initial salvage therapy in 135 patients with progressive, metastatic, testicular germ-cell tumors after initial etoposide-cisplatin–based therapy.16 Approximately 50% of patients achieved a complete response, with a median PFS of 4.7 years, thereby establishing the combination of VeIP as an option for durable complete remissions in second-line metastatic disease.16
TIP
Building on the result of the VeIP trial,16 Memorial Sloan-Kettering Cancer Center (MSKCC) conducted a study substituting paclitaxel for vinblastine; patients with relapsed disease received paclitaxel, ifosfamide, and cisplatin (TIP) for 4 cycles.17 The efficacy of TIP was tested in 46 patients, wherein 70% achieved complete remission and a 2-year PFS of 65%. Myelosuppression was the primary toxicity, with 48% of patients requiring hospitalization for febrile neutropenia. This study was limited by the small number of patients and the lack of a control arm.17
Tandem Stem-Cell Transplant
The outlook for certain patients with testicular-cell tumors of poor prognosis and those harboring cisplatin-refractory disease, defined as progression within 4 weeks of initial cisplatin therapy, is less optimistic. Dose-intensive therapies were viewed as an option to overcome the drawbacks of traditional chemotherapy. However, the dose escalation of the most potent chemotherapy, cisplatin, is limited by nonmyelosuppressive toxicity, such as renal failure. Carboplatin, a second-generation platinum compound that showed activity in testicular germ-cell tumors with a dose-limiting toxicity of myelosuppression, was selected with etoposide for evaluation in the initial dose-intensive escalation followed by autologous stem-cell transplantation (ASCT). A brief experience with 25 patients showed that 52% of patients were disease free, with a median follow-up of 26 months.22
Einhorn and colleagues described 184 consecutive patients with metastatic testicular germ-cell tumors progressing after receiving cisplatin-based chemotherapy in 2007.18 Peripheral blood stem cells were collected, and patients underwent high-dose chemotherapy followed by stem-cell reinfusion for 2 cycles, which is referred to as a “tandem ASCT.” High-dose chemotherapy consisted of 2 cycles of carboplatin 700 mg/m2 and etoposide 750 mg/m2, both given intravenously 5, 4, and 3 days before peripheral blood stem cell infusion.18
At these doses of chemotherapy, most patients will develop severe lower gastrointestinal mucositis, leading to profound diarrhea, and severe prolonged nausea/vomiting, and will require close monitoring of renal function with electrolyte replacement as a result of the high-dose carboplatin and previous cisplatin exposure. For each cycle of high-dose chemotherapy, the minimum requirement was 1 million CD34+ cells per kilogram of body weight. After recovery of granulocyte and platelet counts, the second cycle of high-dose chemotherapy was given, unless a grade 4 nonhematologic toxic effect was present or if there was no response to the first course. The majority of patients who had complete or partial remission after receiving 2 cycles of high-dose chemotherapy and who had normal serum levels of βb-hCG and AFP received a daily maintenance oral dose of 50 mg/m2 etoposide for 21 consecutive days every 4 weeks for 3 cycles.18
Approximately 60% of patients attained complete remission without relapse. At 4 years, of the 135 patients who received the treatment as second-line therapy, approximately 69% attained prolonged disease-free survival compared with approximately 45% of patients who received transplant as third-line or later therapy.18 Of the 40 patients with platinum refractory disease, 45% remained free of disease for a median of 49 months.18 This observation provides data to support that patients with platinum refractory disease can still obtain sustained disease-free survival.
Other published evidence documenting high-dose chemotherapy and stem-cell transplant has included the use of a few cycles of standard-dose chemotherapy followed by high-dose chemotherapy and autologous stem-cell rescue. Pico and colleagues randomized platinum-resistant patients to 4 cycles of cisplatin-, mesna-, and ifosfamide-based chemotherapies (N = 128) or 3 cycles of the same conventional-dose chemotherapy followed by carboplatin 0 mg/m2 to 550 mg/m2 based on creatinine clearance on day 1, etoposide 450 mg/m2, cyclophosphamide 1600 mg/m2, and mesna 3600 mg/m2 for 4 days followed 3 days later by stem-cell reinfusion (N = 135).44 No significant difference was observed between the groups with regard to event-free survival or OS (which was 53% at 3 years).44
Other Combination and Single-Agent Regimens
Feldman and colleagues from MSKCC reported a phase 1/2 trial administering paclitaxel and ifosfamide every 14 days for 2 cycles followed by carboplatin 7 mg/ mL/min to 8 mg/mL/min plus etoposide 400 mg/m2 daily for 3 days and stem-cell rescue every 21 to 28 days for 3 cycles (N = 104).23 The 5-year OS and disease-free survival were 52% and 47%, respectively. Although Einhorn and colleagues18 and Feldman and colleagues23 used high-dose carboplatin and etoposide, the latter included an induction-like regimen, administering 3 rather than 2 high-dose cycles, a targeted area under the curve rather than using mg/m2, and did not use maintenance etoposide.23 High-dose chemotherapy produced durable remissions in more than 50% of patients with relapsed disease, confirming that the procedure is an effective salvage therapy.
Gemcitabine in combination with oxaliplatin has been evaluated in a phase 2 clinical trial and yielded a response rate of 44% in cisplatin-refractory testicular germ-cell tumors.45
The combination of paclitaxel 110 mg/m2 and intravenous gemcitabine 1000 mg/m2 on days 1, 8, and 15 every 28 days has been studied as a salvage regimen.46 Hinton and colleagues conducted a phase 2 trial in patients with refractory testicular germ-cell tumors with the Eastern Cooperative Oncology Group evaluating this regimen in 28 patients.46 The baseline characteristics of this trial included patients whose disease had progressed after 2 previous cisplatin-based regimens or after high-dose chemotherapy, had used 1 previous cisplatin-based regimen with either a mediastinal primary tumor, or had progression within 4 weeks of treatment with cisplatin. Responses were documented in 7 patients, including 3 complete responses, 2 of whom had no evidence of disease at the time of publication. Myelosuppression was the most frequently reported grade 3/4 toxicity, with 56% of patients developing neutropenia, 33% developing thrombocytopenia, and 11% developing anemia.46
In another combination trial of paclitaxel and gemcitabine, Einhorn and colleagues administered the combination to 32 patients with progressive disease after high-dose chemotherapy with ASCT.28 This regimen was third-line therapy in 25 patients, fourth-line therapy in 6 patients, and fifth-line therapy in 1 patient. A complete response was achieved in 6 (19%) patients, with 4 patients remaining disease-free between 20 and 57 months at the time of the study’s publication. A partial response was achieved in 4 patients (12.5%); however, these patients all relapsed within 6 months.28 Novel single agents should continue to be evaluated in this setting for the rare patients who continue to progress despite first-, second-, and third-line therapy.
Toxicities with Treatment
Toxicities are inherent with chemotherapy treatment. Both acute and delayed side effects are associated with the treatment of testicular germ-cell tumors and should be monitored and/or treated based on the toxicity the patient is experiencing (Table 5). Acute toxicities can happen immediately with therapy, and late toxicities are those that persist for more than 12 months after therapy.47 Cisplatin-based chemotherapy is a classically known highly emetogenic chemotherapy regimen, and nausea and vomiting can be devastating if not adequately prevented. Premedication with agents such as 5-HT3 antagonists, steroids, and/or aprepitant are important. Cisplatin can cause renal tubular damage, and it is imperative to appropriately hydrate the patient before and after receiving 5 days of cisplatin therapy.48
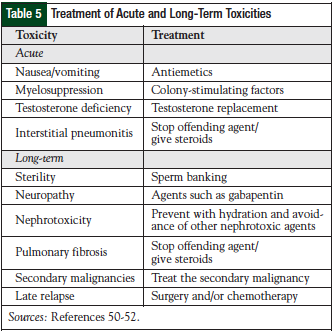
Combination chemotherapy utilized for the treatment of testicular germ-cell tumors can cause myelosuppression. Prophylactic colony-stimulating factors (CSFs) can be used to decrease the risk of febrile neutropenia (eg, pegfilgrastim). If a patient experiences febrile neutropenia, the patient should be treated according to the guidelines put forth by the Infectious Diseases Society of America.49 If the patient experiences neutropenic fever, the chemotherapy dose should not be lowered if the goal of therapy is cure. The use of CSFs should be incorporated to maintain dose intensity and should also be administered in the salvage setting.49 Erythropoietin-stimulating agents should be avoided in this setting, given the curative intent of the chemotherapy treatment.50 Another acute toxicity that a patient may experience is interstitial pneumonitis. This is different from the long-term pulmonary toxicity of pulmonary fibrosis.51-53
Long-term toxicities may include sterility, neuropathy, nephrotoxicity, pulmonary fibrosis, tinnitus, vascular toxicities, secondary malignancies, and late relapses. Sterility is an important adverse event in this patient population, because the men are young and have a long life expectancy. At diagnosis, more than 50% of patients have evidence of impaired spermatogenesis, with approximately 10% to 35% having some degree of infertility.52 Chemotherapy, as well as other modalities of treatment such as abdominal radiation, can induce sterility; sterility can also be a side effect of RPLND.54 After treatment, follicle-stimulating hormone and luteinizing hormone levels can be elevated up to 2 years and can lead to azoospermia. After treatment, men can regain normal sperm counts and father children; however, this is not always certain. Therefore, sperm banking should be considered for males who wish to father children.51
Therapy should not be delayed for an extended period of time to undergo sperm banking, because testicular cancer is a rapid-growing disease when it is not treated.51-54
Neuropathy, which can result from treatment with both cisplatin and vinblastine, is a product of damage to sensory fibers, and causes numbness and tingling in the hands and feet.51-54 These toxicities may improve over time, or the patient may experience long-term problems. High-frequency hearing loss is associated with cisplatin therapy, and patients should be routinely monitored during therapy for changes in hearing.51-54
Pulmonary fibrosis is a classic toxicity associated with bleomycin therapy. Some patients may be more prone to pulmonary toxicity, such as those who smoke, those who have received previous chest radiation, those who have received large doses of bleomycin (>300 units), those who have had previous surgery (intubation), or those who have been exposed to high concentrations of oxygen.51,52 Resultant toxicities can include bronchiolitis obliterans or interstitial pneumonitis. Over time, lung function can be preserved in most patients; however, symptoms can persist in some patients. Treatment involves stopping the offending agent (bleomycin) and the administration of steroids for symptomatic relief. The patient may be evaluated before each dose of chemotherapy with pulmonary function tests to identify if changes need to be made to the bleomycin dosing; however, these tests have not proved to be significantly better in identifying clinical symptoms of pulmonary damage.3 As a late complication, a patient may experience signs of pulmonary fibrosis, unlike the acute toxicity with interstitial pneumonitis.51,52
Vascular toxicities can range from Raynaud’s phenomenon to cardiac problems, such as myocardial infarction (MI), thromboembolic disease, hyperlipidemia, hypertension, stroke, and metabolic syndrome.52,55 Raynaud’s phenomenon has been reported in up to 50% of patients and may persist for years after therapy.53 Bleomycin has traditionally been identified as the offending agent; however, data have demonstrated some association with cisplatin-induced hypomagnesemia.52 No formal treatment is available for this toxicity.
Long-term cardiac toxicities have been identified in this patient population. In a report of 87 patients, 8% developed an MI, ischemia, or cerebrovascular event.55 This may seem like a small number of patients, but these toxicities are not frequently seen in men aged 30 to 42 years.55 Hyperlipidemia and metabolic syndrome have been documented in 80% and 40% of survivors, respectively.51 A full understanding of the mechanism of cardiovascular (CV) disease has not been fully elucidated, but some researchers believe some level of direct vascular injury may occur from radiation or chemotherapy. Other theories suggest that cytokine release, vascular injury, changes in electrolytes, or platelet aggregation may play a role. One report identified survivors of testicular cancer who received cisplatin-based chemotherapy and were 3 times more likely to develop metabolic syndrome compared with the general population.55 Because of these increased risks of CV events, it is important for patients to be appropriately screened and monitored for hypertension, hyperlipidemia, and metabolic syndrome, because education and screening information typically does not occur in this population.
The risk of secondary malignancy is increased after treatment for testicular germ-cell tumors. Compared with the general population, survivors of testicular germ-cell tumors are at an approximately 30% increased risk of secondary malignancies when evaluated in population-based cancer registries over a 40-year time span.52 Among more than 40,000 patients who were followed for 11.5 years, 5.6% developed a secondary malignancy.56 Secondary solid tumors associated with this patient population include cancers of the pleura, pancreas, stomach, bladder, and connective tissue. Leukemia is associated with combination chemotherapy, specifically etoposide and alkylating agents, such as ifosfamide and cyclophosphamide. The risk is increased based on dose. Doses of etoposide >2000 mg/m2 as opposed to doses ≤2000 mg/m2 incur more risk of leukemia (5-year risk, 2% vs 0.5%, respectively).52 Early after therapy, patients are at an increased risk of developing leukemia (3%-7% risk within 10 years of therapy); however, 10 to 20 years after treatment, the risk for leukemia is no different from that in the general population.51
One of the long-term challenges associated with treatment of testicular germ-cell tumors is late relapse. Most patients will recur within 2 years of treatment; however, 2% to 3% of patients will recur after this time period.51,52 In these scenarios, the disease is rarely curable with chemotherapy, and surgical resection is the standard treatment approach because most patients are refractory to chemotherapy.51,52 The most common sites of recurrence are the chest and retroperitoneum. Treatment in this scenario will depend on the previous therapies. Although almost all patients can be cured of their disease, many men struggle with long-term toxicities associated with therapy. Routine evaluation/screening should be conducted to evaluate for vascular, renal, otologic, neurologic, and reproductive toxicities associated with therapy.
With the growing knowledge of long-term toxicities in this patient population (even after curing these individuals), it is important to understand the need for long-term follow-up to monitor the previously mentioned toxicities, and also the psychosocial morbidity associated with the diagnosis and treatment effects.51-53 The psychosocial needs can be related to fatigue, mental health, sexuality, employment, cognitive impairment, and quality of life. Institutions should have practice-based guidelines established to help monitor these patients throughout their life.
Practical Clinical Questions
Question 1. How should we deal with chemotherapy drug shortages?
Many pharmacies today are faced with the recurring problem of drug shortages. In most instances, there are alternate therapies for drug substitutions, but in the case of testicular germ-cell tumor treatment, this has been a dilemma that pharmacists and physicians have faced when chemotherapy has not been available. In 2010, bleomycin, carboplatin, cisplatin, and etoposide were all reported as being in short supply. In the fall of 2010, etoposide was on national backorder, and many centers faced an acute shortage of the drug that persisted for several months. This is a disease for which treatment should not be delayed because of lack of a drug. Treatment delays can directly compromise the potential for cure, because of the high growth index of the tumor and the inherent sensitivity to chemotherapy.
In patients deemed to be good risk by the IGCCCG (Table 2), PVB (Table 3) can be substituted for BEP. This regimen was the standard of care for the treatment of testicular germ-cell tumors in the 1980s before being compared with BEP.13 Toxicity was reported to be higher with the PVB regimen, including neuromuscular toxicity (eg, paresthesias, abdominal pain, myalgias), so patients must be counseled accordingly.16 In poor-risk patients, VIP (Table 4) can be substituted for BEP. This regimen has been studied extensively in the salvage/relapse setting.16 Institutions should have a plan as to how to prioritize patients based on their drug supply, disease states, and the patient’s goal of therapy. If a facility is completely out of stock of one of these critical agents, every effort should be made to locate another site where a patient can receive complete treatment.
Question 2. How should cisplatin be dosed in patients with underlying dysfunction?
Cisplatin causes renal tubular damage from the metabolism via glutathione conjugates and the production of reactive oxygen species, leading to proximal tubular damage.57 Patients are adequately hydrated before and after treatment to mitigate this toxicity. However, some patients may have preexisting renal dysfunction at baseline. The most important and most active drug of any of the chemotherapy regimens for testicular germ-cell tumors is cisplatin. With cure as the goal of treatment in this patient population, the decision has to be made to balance the toxicities of cisplatin, most of which are reversible, with the potential for compromising cure with any dose reduction of cisplatin. At our institution, we do not dose-adjust cisplatin based on one’s renal dysfunction. The most active drug of all of those discussed for the treatment of testicular cancer is cisplatin. By dose-reducing cisplatin, one could potentially compromise a patient’s success in being cured of the disease. The patients should be closely monitored in terms of laboratory values and input/output, and should receive adequate hydration before and after chemotherapy to protect from any further renal effects.
Question 3. Is a bleomycin test dose really needed?
This is an age-old question that continues to circulate among clinicians. The occurrence of hypersensitivity reactions has been reported; however, these reports date back to the 1970s. In a review by Lam, the question was asked, “Do we need routine bleomycin test dosing in the 21st century?”24 Based on the review of the literature, the recommendation is not to administer a test dose, because of the unpredictable onset of hypersensitivity and the timing of the reaction. At our institution, we do not administer a test dose to patients who are receiving bleomycin therapy.
Conclusion
With overall cure rates of more than 90%, testicular germ-cell tumors are the model of care for curable cancer. Patients with refractory or relapsed disease after initial therapy still have the potential for cure with second- and third-line chemotherapies with cisplatin-based regimens or with high-dose chemotherapy followed by ASCT. Pharmacists caring for this patient population must monitor for common acute toxicities of the standard chemotherapy regimens for and long-term complications with cured patients, most of whom can live a normal life.
Author Disclosure Statement
Dr Kiel, Dr Fausel, and Dr Jones reported no conflicts of interest.
References
- Siegel R, Ward E, Brawley O, Jemal A. Cancer Statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer death. CA Cancer J Clin. 2011;61:212-236.
- Devesa SS, Blot WJ, Stone BJ, et al. Recent cancer trends in the United States. J Natl Cancer Inst. 1995;87:175-182.
- Bosl GJ, Bajorin DF, Sheinfeld J, et al. Cancer of the testis. In: Devita VT, Lawrence TS, Rosenberg SA, et al, eds. Cancer: Principles and Practice of Oncology. Philadelphia, PA: Lippincott Williams and Wilkins; 2008:1463-1485.
- Eble J, Sauter G, Epstein J, et al. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs (IARC WHO Classification of Tumours). Lyons, France: International Agency for Research on Cancer Press; 2004.
- Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer Verlag; 2002.
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Consensus Classification Group. J Clin Oncol. 1997;15:594-603.
- Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97:1869-1875.
- Li MC, Whitmore WF Jr, Golbey R, Grabstald H. Effects of combined drug therapy on metastatic cancer of the testis. JAMA. 1960;174:1291-1299.
- Samuels ML, Lanzotti VJ, Holoye PY, et al. Combination chemotherapy in germinal cell tumors. Cancer Treat Rev. 1976;3:185-204.
- Einhorn LH, Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med. 1977;87:293-298.
- Williams SD, Birch R, Einhorn LH, et al. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435-1440.
- Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
- Saxman SB, Finch D, Gonin R, Einhorn LH. Long-term follow-up of a phase III study of three versus four cycles of bleomycin, etoposide, and cisplatin in favorable-prognosis germ-cell tumors: the Indiana University experience. J Clin Oncol. 1998;16:702-706.
- Culine S, Kerbrat P, Kramar A, et al. Refining the optimal chemotherapy regimen for good-risk metastatic nonseminomatous germ-cell tumors: a randomized trial of the Genito-Urinary Group of the French Federation of Cancer Centers (GETUG T93BP). Ann Oncol. 2007;18:917-924.
- Loehrer PJ Sr, Lauer R, Roth BJ, et al. Salvage therapy in recurrent germ cell cancer: ifosfamide and cisplatin plus either vinblastine or etoposide. Ann Intern Med. 1988;109:540-546.
- Loehrer PJ Sr, Gonin R, Nichols CR, et al. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol. 1998;16:2500-2504.
- Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549-6555.
- Einhorn LH, Williams SD, Chamness A, et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med. 2007;357:340-348.
- Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:
242-254. - de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol. 2001;19:1629-1640.
- de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828-832.
- Broun ER, Nichols CR, Gize G, et al. Tandem high dose chemotherapy with autologous bone marrow transplantation for initial relapse of testicular germ cell cancer. Cancer. 1997;79:1605-1610.
- Feldman DR, Sheinfeld J, Bajorin DF, et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol. 2010;28:1706-1713.
- Lam MS. The need for routine bleomycin test dosing in the 21st century. Ann Pharmacother. 2005;39:1897-1902.
- Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A. 2002;99:4592-4595.
- Higby DJ, Wallace HJ Jr, Albert DJ, Holland JF. Diaminodichloroplatinum: a phase I study showing responses in testicular and other tumors. Cancer. 1974;33:1219-1225.
- Bedano PM, Brames MJ, Williams SD, et al. Phase II study of cisplatin plus epirubicin salvage chemotherapy in refractory germ cell tumors. J Clin Oncol. 2006;24:5403-5407.
- Einhorn LH, Brames MJ, Juliar B, Williams SD. Phase II study of paclitaxel plus gemcitabine salvage chemotherapy for germ cell tumors after progression following high-dose chemotherapy with tandem transplant. J Clin Oncol. 2007;
25:513-516. - Jones WG, Fossa SD, Mead GM, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I testicular seminoma: a report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328). J Clin Oncol. 2005;23:
1200-1208. - Alomary I, Samant R, Gallant V. Treatment of stage I seminoma: a 15-year review. Urol Oncol. 2006;24:180-183.
- Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957-962.
- Beck SD, Bey AL, Birhle R, Foster RS. Ejaculatory status and fertility rates after primary retroperitoneal lymph node dissection. J Urol. 2010;184:2078-2080.
- de Wit R, Fizazi K. Controversies in the management of clinical stage I testis cancer. J Clin Oncol. 2006;24:5482-5492.
- Albers P, Siener R, Krege S, et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966-2972.
- Culine S, Theodore C, Terrier-Lacombe MJ, Droz JP. Primary chemotherapy in patients with nonseminomatous germ cell tumors of the testis and biological disease only after orchiectomy. J Urol. 1996;155:1296-1298.
- Davis BE, Herr HW, Fair WR, Bosl GJ. The management of patients with nonseminomatous germ cell tumors of the testis with serologic disease only after orchiectomy. J Urol. 1994;152:111-113; discussion 114.
- Steyerberg EW, Keizer HJ, Fosså SD, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol. 1995;13:1177-1187.
- Beck SD, Foster RS, Bihrle R, et al. Outcome analysis for patients with elevated serum tumor markers at postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol. 2005;23:6149-6156.
- Albers P, Ganz A, Hannig E, et al. Salvage surgery of chemorefractory germ cell tumors with elevated tumor markers. J Urol. 2000;164:381-384.
- Williams SD, Einhorn LH, Greco FA, et al. VP-16-213 salvage therapy for refractory germinal neoplasms. Cancer. 1980;46:2154-2158.
- Hainsworth JD, Williams SD, Einhorn LH, et al. Successful treatment of resistant germinal neoplasms with VP-16 and cisplatin: results of a Southeastern Cancer Study Group trial. J Clin Oncol. 1985;3:666-671.
- Wheeler BM, Loehrer PJ, Williams SD, Einhorn LH. Ifosfamide in refractory male germ cell tumors. J Clin Oncol. 1986;4:28-34.
- Loehrer PJ Sr, Einhorn LH, Williams SD. VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J Clin Oncol. 1986;4:528-536.
- Pico JL, Rosti G, Kramar A, et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol. 2005;16:1152-1159.
- Kollmannsberger C, Beyer J, Liersch R, et al. Combination chemotherapy with gemcitabine plus oxaliplatin in patients with intensively pretreated or refractory germ cell cancer: a study of the German Testicular Cancer Study Group. J Clin Oncol. 2004;22:108-114.
- Hinton S, Catalano P, Einhorn LH, et al. Phase II study of paclitaxel and gemcitabine in refractory germ cell tumor (E9897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2002;20:1859-1863.
- Fosså SD, Gilbert E, Dores GM, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst. 2007;99:533-544.
- Chabner BA, Amrein PC, Druker BJ, et al. Chemotherapy of neoplastic diseases. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006:
1315-1403. - Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56-e93.
- Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/
American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116:
4045-4059. - Pliarchopoulou K, Pectasides D. Late complications of chemotherapy in testicular cancer. Cancer Treat Rev. 2010;36:262-267.
- Travis LB, Beard C, Allan JM, et al. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010;102:1114-1130.
- Abouassaly R, Fosså SD, Giwercman A, et al. Sequelae of treatment in long-term survivors of testis cancer. Eur Urol. 2011;60:516-526.
- Vaughn DJ, Gignac GA, Meadows AT. Long-term medical care of testicular cancer survivors. Ann Intern Med. 2002;136:463-470.
- Redig AJ, Munshi HG. Metabolic syndrome after hormone-modifying therapy: risks associated with antineoplastic therapy. Oncology (Williston Park). 2010;24:
839-844. - Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
- Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47-61.
