Malignancy has long been recognized as a risk factor for venous thromboembolism (VTE), dating back to the 19th century, when Armand Trousseau diagnosed the syndrome on himself.1 Although the risk for thromboembolism varies based on patients’ cancer type overall, it has been estimated that patients with cancer have a 4- to 7-fold higher risk for thromboembolism than the general population.1-3 Tumor production of hypercoagulable substances (eg, tissue factor and tumor compression of blood vessels) may explain part of the increased risk for VTE associated with malignancy.4 In addition, patients with cancer often have indwelling catheters and receive medications (eg, tamoxifen, bevacizumab, cisplatin, lenalidomide) that increase the risk for thrombosis.5,6
Low-molecular-weight heparin (LMWH) and sulfated non–anticoagulant LMWH have been shown to suppress tumor growth with and without traditional chemotherapeutic agents.7 In addition, intracellular processes affecting cancer cell adhesion and migration have been shown to be inhibited with LMWH products.8-12 Enoxaparin has also been shown to suppress cell proliferation in adenocarcinomic epithelial cell lines.13 Because of these targeted effects on specific cancer cells, LMWH derivatives are being evaluated not only for their therapeutic effects, but also as drug delivery vehicles for chemotherapy.14,15
Clinically, LMWH has consistently been shown to decrease rates of recurrent VTE with similar or lower rates of major bleeding compared with traditional vitamin K antagonist (VKA) therapy.16-20 LMWH may also positively impact survival, particularly for patients with less advanced disease progression, presenting a more favorable prognosis.10,21-25 Based on these findings, contemporary guidelines have reached a consensus that patients with cancer should be treated with LMWH long term when diagnosed with VTE.26-28
Despite a large body of evidence, there is still reluctance by physicians to follow guidelines because of subjective fears regarding exposing patients to LMWH for a prolonged period.29,30 Two large studies of patients with cancer and VTE, which were based on 2 large databases—the Multicenter Advanced Study for a Thromboembolism Registry (MASTER) and the Computerized Registry of Patients with Venous Thromboembolism (RIETE)—featuring a substantial subgroup of patients with cancer (approximately 20% in each registry), noted that significantly more patients with cancer were treated with LMWH than patients without cancer. Those rates, however, were only 30% and 50%, respectively, compared with patients receiving VKA therapy.31,32
To evaluate the dissemination of evidence-based, clinical recommendations in a community teaching setting, we designed a study to evaluate the prescribing rates of LMWH for the long-term management of VTE in patients with active cancer.
Methods
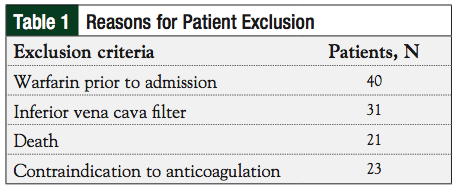
This study was conducted in the United States at a single, regional, referral community teaching hospital with an affiliated regional cancer center located in a rural area. After receiving local institutional review board approval, medical records from January 1, 2005, to December 31, 2009, that were listed with an International Classification of Diseases, Ninth Revision (ICD-9) code for any cancer type and a concomitant ICD-9 code for any VTE, were identified and reviewed. Patients were excluded if they received warfarin prior to admission, were given an inferior vena cava filter, were pregnant, had a documented contraindication to anticoagulation at the time of admission, or died during hospitalization. Patients with duplicate events were only included for their initial event.
Additional data collected included patient demographics; cancer type; VTE type; length of time since cancer diagnosis; serum creatinine measurements; international normalized ratio; platelet count at discharge; history of thrombocytopenia or heparin-induced thrombocytopenia (HIT); specialty of the prescriber; and bleeding events prior to or during admission.
The principal outcome of this study was the primary maintenance of anticoagulant prescribed at the time of discharge. LMWH prescribed as a short-term bridge for chronic warfarin therapy was classified as warfarin (VKA group), and LMWH therapy prescribed as the primary maintenance regimen was classified as LMWH (LMWH group). Secondary outcomes included specialty of the prescriber, adverse bleeding events during hospitalization, and whether a contraindication to anticoagulant therapy existed at the time of discharge.
Patients were considered to have an adverse bleeding event if their hemoglobin decreased by more than 2 g/dL in any one 24-hour period during admission, if they required a transfusion of packed red blood cells, or if an adverse bleeding event requiring increased physician monitoring was documented. Patients were considered to have a contraindication to anticoagulant therapy if the provider stated so at the time of discharge, regardless of clinical reasoning.
The binomial test was used to evaluate anticoagulant prescribed at the time of discharge expecting a conservative 70% prescribing rate for warfarin (30% for LMWH). Logistic regression analysis was used to evaluate the correlation of the type of discharging physician with the prescribing of LMWH or warfarin at the time of discharge. Chi-square, Fisher exact test, and the student’s t-test were used when appropriate to evaluate baseline patient demographics. Data analyses were completed using IBM SPSS Statistics, version 21 (IBM Corporation, Armonk, NY).
Results
Of the 244 patients identified based on the inclusion criteria, 115 were excluded (Table 1); 129 patients were included in the study analysis. One hundred seven patients were prescribed warfarin, or warfarin plus LMWH at discharge. Nine patients were prescribed LMWH as the primary treatment for their VTE at time of discharge, and 13 patients were not prescribed any anticoagulants.
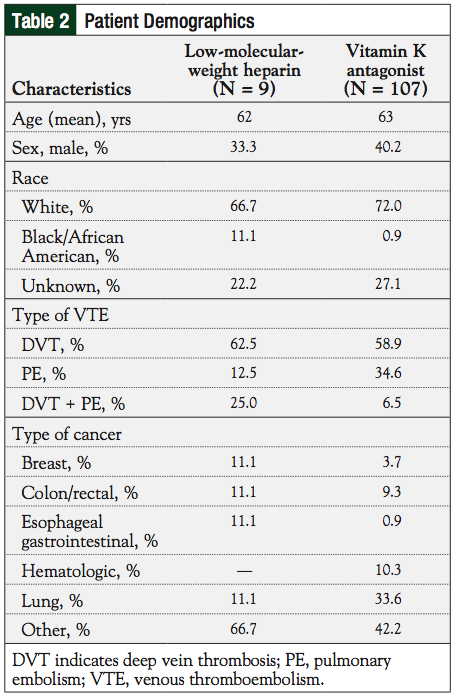
The mean age of patients was 62 and 63 years in the LMWH and VKA groups, respectively (Table 2). There were no statistical differences with any variables between the 2 study groups.
Two patients prescribed warfarin had either HIT or a positive HIT antibody test during the time of admission. Of the 9 patients who received LMWH, 1 patient was documented to have “refused warfarin” at the time of their discharge. One patient who received neither LMWH nor warfarin was documented as “intolerant to warfarin”; however, no other contraindications to LMWH or any other anticoagulants were noted.
Significantly fewer patients (n = 9; 7.40%) were prescribed LMWH as their primary anticoagulant at the time of discharge compared with warfarin (n = 107 [82.9%]; P <.0001). Of the patients prescribed LMWH at discharge, 4 (44.4%) were prescribed LMWH by a hematologist/oncologist, and 3 by general practitioners. Only 7 of the 107 patients (6.5%) who were prescribed warfarin were under the care of a hematologist/oncologist. Hematologists/oncologists were therefore 7.8 times more likely (P = .016) to prescribe LMWH than warfarin compared with any general practitioner based on logistic regression analysis of practice specialty in regard to prescribing of LMWH versus VKA (Table 3).
Seven patients had documented bleeding during admission with only 2 patients requiring transfusion. Four patients were prescribed warfarin and 1 patient was prescribed LMWH for the treatment of VTE at the time of discharge.
Discussion
A frequently hypothesized reason for the discrepancies between prescribing more efficacious LMWH in patients with cancer and VTE is the increase in cost.33 For patients without prescription coverage, generic warfarin can be obtained for merely a few dollars each month, whereas LMWH would cost patients thousands of dollars for a 6-month treatment period. For patients without prescription coverage, cost would be an inhibitive deterrent to obtaining the more effective LMWH treatment. Conversely for those with prescription coverage, patient cost may be less of an issue.
A cost-effectiveness analysis published in 2005 found that LMWH provided a quality-adjusted life expectancy of 1.097 quality-adjusted life-years with a cost of $15,329.33 The vast majority of the cost were attributed to pharmacy costs for LMWH. Although the LMWH enoxaparin is now available as a generic, its costs are still high compared with warfarin therapy. With the early mortality benefit associated with LMWH, it is likely prudent to still preferentially consider it in accordance with current guidelines, when the patient has prescription coverage to offset the drug acquisition cost.
It is well known by clinicians that there is substantial lag time between the publication of new research findings and their incorporation into everyday clinical practice. A literature search using PubMed and the Internet, yielded little to no information regarding the typical lag time from publication, guideline incorporation or bedside use. Several researchers have attempted to measure the time from translational research to bedside implementation, but there is no consensus on how to define and measure this process.34 Even measuring the uptake of clinical trial results leads researchers down the road of counting publications and citations,35 which has little to no meaning for those trying to answer the question of true dissemination to bedside clinical practice.
We present data on a treatment approach that is clearly known to be beneficial. The Chest antithrombotic guidelines incorporated a recommendation to use LMWH for the initial 3 to 6 months after the diagnosis of acute VTE in patients with cancer in the 2004 iteration of their guidelines.36 Contemporary papers have been written on this issue with directives on how to improve adherence to guidelines29,37; however, there is still a disconnect between adherence to long-standing evidence-based recommendations and actual clinical practice.
Previous studies have demonstrated that a large proportion, and in some instances the majority of prescribers, do not adhere to the recommendation to use LMWH for the initial acute treatment of VTE in patients with active cancer. LMWH prescribing rates increased from 32% to 60%, approximately, during the 3 to 5 year period following the 2004 CHEST guidelines in a Canadian patient population.38-40 Similar to our study, Rahme and colleagues observed a higher rate of LMWH prescribing among oncologists compared with general practitioners; however, their logistic regression model did not show a significant difference40 as did our study. This is likely due to the higher rate (59%) of prescribing in their study by general internists/practitioners compared with no LMWH prescribed by generalists in our study.
The complexity of this issue drove Johnson and colleagues to evaluate the reasons physicians may not adhere to the recommendation to prescribe LMWH for these patients.30 Key findings were that physicians struggled with the appropriateness of this recommendation with concerns ranging from increased patient burden to individual patient prognosis. Although physicians may not adhere to this recommendation for a variety of reasons, the result is the same: the patient often receives warfarin instead of LMWH despite the evidence favoring the latter.
Despite these known barriers, we expected to see higher rates of LMWH prescriptions in our cohort in a community teaching hospital, but the rates of LMWH prescribing for acute VTE in patients with active cancer were abysmally low. This study indicates that specialists (oncologists) who should be more familiar with evidence-based recommendations in patients with cancer did indeed prescribe LMWH almost 8-fold more often than their general practitioner colleagues. A qualitative study looking at the way physicians make decisions in this patient population also identified a similar trait among oncologists compared with general practitioners.30 This could also be explained by specialists being more likely to exhibit traits of an “innovator” or “early adopter” as defined by Berwick,41 suggesting that they are more likely to be early utilizers of novel therapies.
Although previous registry data have estimated substantially higher LMWH prescribing rates in this population,31,32 our data may more accurately represent “real- world” prescribing rates in a typical, community American healthcare setting, particularly in rural areas. There are real barriers to prescribing LMWH that include access and medication acquisition cost that are difficult to identify, even in qualitative “think aloud” studies. These types of health disparities, such as access to specialists and prescription drug coverage, are observed more often in rural areas.42 Likewise, these data are consistent with the observation by Rahme and colleagues that showed a correlation between residing in a rural area and being prescribed VKAs instead of LMWH.40
Limitations
The limitations of our current study include the retrospective nature of the observations that were recorded. In addition, our LMWH group was small and comprised only 7% of our total patient population. Taking the primary outcome we evaluated into consideration, this demonstrated a significant difference in prescribing choice for our patient population. The cancer type was more widely distributed, which prevented any meaningful interpretations between the malignancy type and prescribing patterns of physicians. Although this is a limitation, given that some malignancies have a much higher rate of thrombosis than others, it is unlikely this weighs on the decision of prescribers because clinicians may disregard evidence-based guideline recommendations.
Conclusion
We evaluated prescribing patterns of physicians in regard to evidence-based treatment recommendations for patients with active cancer and acute deep vein thrombosis and/or pulmonary embolism.
Similar to previous studies, we observed a very low rate of adherence to guideline recommendations, which was more pronounced in our rural geographic area than in previous Canadian studies. To our knowledge, contemporary data in a US patient population are not available, which suggests the possibility that this large discrepancy may not be limited to only our rural area.
Evidence demonstrates that patients with cancer and acute VTE treated with LMWH have a lower recurrence of VTE, lower mortality rates, and decreased adverse bleeding when compared with traditional warfarin therapy. Hematologists and oncologists are more likely than their generalist colleagues to follow evidence-based treatment guidelines when prescribing LMWH in this patient population.
Acknowledgments
The authors thank Jarrett Wyatt, PharmD (student pharmacist at time of data collection), for his assistance in data collection and entry. The abstract was presented in poster format at the ACCP Virtual Poster Forum May 2013.
Author Disclosure Statement
Dr Stewart serves on the Speaker’s Bureau for Janssen Pharmaceuticals. Dr Rikhye, Dr Odle, Dr Bossaer, and Dr Flores reported no conflicts of interest.
Drs Stewart, Odle, and Flores are Associate Professors of Pharmacy Practice, and Dr Bossaer is Assistant Professor of Pharmacy Practice, Bill Gatton College of Pharmacy, East Tennessee State University, Johnson City, TN; Dr Rikhye is an Emergency Medicine Physician, Professional Emergency Physician Services, Oakland, MD.
References
1. Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723-1729.
2. Dammacco F, Vacca A, Procaccio P, et al. Cancer-related coagulopathy (Trousseau’s syndrome): review of the literature and experience of a single center of internal medicine. Clin Exp Med. 2013;13:85-97.
3. Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809-815.
4. Davila M, Amirkhosravi A, Coll E, et al. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008; 6:1517-1524.
5. Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab. JAMA. 2008;300:2277-2285.
6. Starling N, Rao S, Cunningham D, et al. Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum, and fluoropyrimidine combination chemotherapy: a report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group. J Clin Oncol. 2009; 27:3786-3793.
7. Phillips PG, Yalcin M, Cui H, et al. Increased tumor uptake of chemotherapeutics and improved chemoresponse by novel non-anticoagulant low molecular weight heparin. Anticancer Res. 2011;31:411-420.
8. Carmazzi Y, Iorio M, Armani C, et al. The mechanisms of nadroparin-mediated inhibition of proliferation of two human lung cancer cell lines. Cell Prolif. 2012;45: 545-556.
9. Chalkiadaki G, Nikitovic D, Katonis P, et al. Low molecular weight heparin inhibits melanoma cell adhesion and migration through a PKCa/JNK signaling pathway inducing actin cytoskeleton changes. Cancer Lett. 2011;312:235-244.
10. Lee AYY. The effects of low molecular weight heparins on venous thromboembolism and survival in patients with cancer. Thromb Res. 2007;120(suppl 2): S121-S127.
11. Castelli R, Porro F, Tarsia P. The heparins and cancer: review of clinical trials and biological properties. Vasc Med. 2004;9:205-213.
12. Maraveyas A, Ettelaie C, Echrish H, et al. Weight-adjusted dalteparin for prevention of vascular thromboembolism in advanced pancreatic cancer patients decreases serum tissue factor and serum-mediated induction of cancer cell invasion. Blood Coag Fibrinol. 2010;21:452-458.
13. Arab WA, Kotb R, Sirois M, et al. Concentration- and time-dependent effects of enoxaparin on human adenocarcinomic epithelial cell line A549 proliferation in vitro. Can J Physiol Pharmacol. 2011;89:705-711.
14. Hou L, Yao J, Zhou J, et al. Pharmacokinetics of a paclitaxel-loaded low molecular weight heparin-all-trans-retinoid acid conjugate ternary nanoparticulate drug delivery system. Biomaterials. 2012;33:5431-5440.
15. Hou L, Fan Y, Yao J, et al. Low molecular weight heparin-all-trans-retinoid acid conjugate as a drug carrier for combination cancer chemotherapy of paclitaxel and all-trans-retinoid acid. Carbohydr Polym. 2011;86:1157-1166.
16. Lee AYY, Levine MN, Baker RI, et al. Low-molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153.
17. Lee AYY. Treatment of established thrombotic events in patients with cancer. Thromb Res. 2012;129(suppl 1):S146-S153.
18. Romera A, Cairols MA, Vila-Coll R, et al. A randomised open-label trial comparing long-term sub-cutaneous low-molecular-weight heparin compared with oral-anticoagulant therapy in the treatment of deep venous thrombosis. Eur J Vasc Endovasc Surg. 2009;37:349-356.
19. Hull RD, Townshend G. Long-term treatment of deep-vein thrombosis with low-molecular-weight heparin: an update of the evidence. Thromb Haemost. 2013; 110:14-22.
20. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer. Arch Intern Med. 2002;162:1729-1735.
21. Noble S. Low-molecular-weight heparin and survival in lung cancer. Thromb Res. 2012;129(suppl):S114-S118.
22. Saraiya B, Goodin S. Management of venous thromboembolism and the potential to impact overall survival in patients with cancer. Pharmacother. 2009;29:1344-1356.
23. Lee AY, Rickles FR, Julian JA, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123-2129.
24. Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol. 2004;22:1944-1948.
25. Klerk CPW, Smorenburg SM, Otten H-M, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005; 23:2130-2135.
26. Mandalà M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22 (suppl 6):vi85-vi92.
27. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189-2204.
28. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2): e419S-e494S.
29. Debourdeau P, Beckers M, Gerome P, et al. How to improve the implementation of guidelines on cancer-related thrombosis. Expert Rev Anticancer Ther. 2011;11:473-483.
30. Johnson MJ, Sheard L, Maraveyas A, et al. Diagnosis and management of people with venous thromboembolism and advanced cancer: how do doctors decide? A qualitative study. BMC Med Inform Decis Mak. 2012;12:75.
31. Imberti D, Agnelli G, Ageno W, et al. Clinical characteristics and management of cancer-associated acute venous thromboembolism: findings from the MASTER Registry. Haematologica. 2008;93:273-278.
32. Monreal M, Falgá C, Valdés M, et al. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2006;4:1950-1956.
33. Aujesky D, Smith KJ, Cornuz J, et al. Cost-effectiveness of low-molecular-weight heparin for secondary prophylaxis of cancer-related venous thromboembolism. Thromb Haemost. 2005;93:592-599.
34. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510-520.
35. Rosas SR, Schouten JT, Cope MT, et al. Modeling the dissemination and uptake of clinical trials results. Res Eval. 2013;22:179-186.
36. Büller HR, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):401s-428s.
37. Kaatz S, Qureshi W, Lavender RC. Venous thromboembolism: what to do after anticoagulation is started. Cleve Clin J Med. 2011;78:609-618.
38. Cook LM, Kahn SR, Goodwin J, et al. Frequency of renal impairment, advanced age, obesity and cancer in venous thromboembolism patients in clinical practice. J Thromb Haemost. 2007;5:937-941.
39. Kahn SR, Springmann V, Schulman S, et al. Management and adherence to VTE treatment guidelines in a national prospective cohort study in the Canadian outpatient setting. Thromb Haemost. 2012;108:493-498.
40. Rahme E, Feugère G, Sirois C, et al. Anticoagulant use in patients with cancer associated venous thromboembolism: a retrospective cohort study. Thromb Res. 2013;131:210-217.
41. Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969-1975.
42. Ricketts TC. The changing nature of rural health care. Annu Rev Public Health. 2000;21:639-657.