It is estimated that in 2014 there will be 15,720 new cases of chronic lymphocytic leukemia (CLL) and 4600 deaths from CLL,1 making it the second most common leukemia in adults in the United States.2 The average age at diagnosis is approximately 72 years.1 CLL is a lymphoproliferative disorder that is characterized by progressive accumulation and subsequent overgrowth of functionally incompetent lymphocytes, which ultimately overcrowd and obstruct the normal processes of healthy B-cell lymphocytes. CLL is nearly identical to the indolent small B-cell lymphoma, except that it is predominately manifested in the bone marrow, whereas B-cell lymphoma is evident more so in the peripheral lymph nodes.3,4 Approximately 25% to 50% of patients with CLL are asymptomatic at the time of diagnosis.5 It is often first detected because of the presence of lymphadenopathy or lymphocytosis.5
The prognosis of CLL is dependent on many factors, including the presence of lymphadenopathy, degree of hepatosplenic involvement, anemia and thrombocytopenia, serum markers (thymidine kinase and β-2 microglobulin), and genetic mutations or cytogenetic abnormalities (eg, deletions of 11q, 13q, and 17p, CD38 and ZAP-70 expression). Patients’ survival times vary considerably, ranging from 2 to 20 years and are dependent on staging and on when treatment is initiated.6,7 The median survival is more than 10 years,6 and the 5-year overall survival is 82%.8 Not all patients with CLL require treatment, because several subsets of patients with this disease may have a normal survival rate.
The clinical features of CLL include the expression of several abnormal B-cell surface antigens, including CD5, CD19, CD20, CD23, and CD52. Both CD20 and CD52 antigens serve as targets for drug therapy.9 Before the recent approval of the monoclonal antibody obinutuzumab, there were 3 other monoclonal antibodies approved for the treatment of CLL, including rituximab, alemtuzumab, and ofatumumab.
Rituximab, a chimeric monoclonal antibody that targets the CD20 antigen on B-cell lymphocytes, is a first-generation anti-CD20 monoclonal antibody that has been shown to improve overall survival in patients with advanced CLL. It was approved by the US Food and Drug Administration (FDA) in 1997 for the treatment of patients with B-cell non-Hodgkin lymphomas, and in 2010 for the treatment of previously treated or untreated patients with CLL.4,10 There have been great efforts to develop new biologic therapies to improve on the efficacy of rituximab and overcome resistance of the CD20 antigen.
Alemtuzumab, a humanized monoclonal antibody that targets the CD52 antigen on lymphocytes, was approved in 2001 under the FDA’s accelerated approval process.11 In 2007, the FDA granted approval for alem-tuzumab to be used as a first-line single-agent option for patients with CLL.4,11
Ofatumumab, a human monoclonal antibody, targets the CD20 antigen on B-cell lymphocytes and was approved by the FDA in 2009 for the treatment of patients with CLL that is no longer responding to available therapies.4,12 In 2014, ofatumumab was approved in combination with chlorambucil for the treatment of previously untreated patients with CLL for whom fludar-abine-based therapy is inappropriate.13
According to the National Comprehensive Cancer Network (NCCN) guidelines for CLL, the initiation of treatment is based on the Rai clinical staging system.4 This system stages a patient based on a physical exam, which includes a complete blood count, an examination of the lymph nodes, and an examination for the presence of organomegaly.14 Very early stage 0 or 1 disease is usually managed by monitoring the patient’s clinical findings and laboratory tests. Active treatment is usually instituted for later stages of the disease or with the presence of any of several conditions, including disease-related symptoms (fever, night sweats, substantial fatigue), painful spleen, 50% increase in lymphocytosis, progressive anemia, thrombocytopenia, splenomegaly, or lymphadenopathy.14
Treatment of CLL
Traditionally, symptomatic CLL has been treated with one of several drugs, including purine analogs (eg, fludarabine, pentostatin), alkylating agents (eg, chlor-ambucil, cyclophosphamide, bendamustine), monoclonal antibodies (eg, rituximab, ofatumumab, alemtuzu-mab), or combinations of these drugs.4
Before the approval of obinutuzumab, the first-line therapy for elderly patients (aged ≥70 years) or frail and without cytogenetic abnormalities, was chlorambucil with or without rituximab. Younger patients aged <70 years received regimens such as fludarabine, cyclophosphamide, and rituximab or bendamustine with or without rituximab.15 Patients with relapsed or refractory disease were treated, retreated, or switched to biologics, such as ofatumumab alone or alemtuzumab with or without rituximab.15
Currently, the preferred first-line therapy for patients aged ≥70 years without cytogenetic abnormalities is obinutuzumab with chlorambucil.4 To guide the selection of all therapy, further testing is required to detect cytogenetic abnormalities.4 These abnormalities are determined through fluorescence in situ hybridization (FISH). Detection of deletions 11q, 13q, or 17p through FISH helps to determine a patient’s prognosis and helps to narrow the therapeutic options.4 Patients with deletion 13q have the most favorable prognosis, with the longest median survival of 133 months.4 Patients with this deletion do not have a specified treatment guideline.4 However, patients who have deletion 11q or 17p have a less favorable prognosis.16 Patients who have deletion 11q have more favorable outcomes when regimens include an alkylating agent.4
Before the approval of obinutuzumab, the first-line therapy for patients with deletion 11q was chlorambucil with or without rituximab.15 The current preferred first-line therapy for patients with deletion 11q is obinutuz-umab with chlorambucil.4 Extensive lymphadenopathy and disease progression are associated with deletion 11q, leading to a median survival of 79 months.16,17
Deletion 17p is associated with low response rates; patients with deletion 17p are recommended to participate in clinical trials if there is no standard therapy.4 If therapy is initiated, a choice of obinutuzumab with chlor-ambucil, high-dose methylprednisolone with rituximab, or alemtuzumab with or without rituximab is preferred as the first-line biologic option.4 In patients with deletion 17p, the median survival time is 32 months.16
Rituximab, given in combination with chemotherapy, can result in significant clinical improvement and, in many cases, long-term survival.18 Rituximab has played a key role in the treatment of B-cell malignancies. It is a very effective drug in the treatment of CLL; however, resistance is a common problem.19 There have been great efforts to develop biologic therapies similar to ri-tuximab with slight modifications to improve the efficacy and overcome resistance to the CD20 antigen.20
The remainder of this concise review is focused on the clinical use of obinutuzumab, a new medication that attacks CD20, and the clinical studies that have led to the approval of this agent for the treatment of patients with CLL.
Obinutuzumab
The Food and Drug Administration Safety and Innovation Act, signed on July 9, 2012, is intended to expedite the development and review of drugs for serious or life-threatening conditions through the request for breakthrough therapy designation.21 Breakthrough therapy–designated drugs must have shown a substantial improvement of outcomes over current therapies.21 Obinutuzumab, a humanized, glycoengineered type II CD20 monoclonal antibody, is the first cancer drug with the breakthrough therapy designation to receive FDA approval.22 On November 1, 2013, the FDA approved obinutuzumab for the treatment of patients with previously untreated CLL. Obinutuzumab was also designated as an orphan drug, because CLL is a rare disease.22
Obinutuzumab Pharmacology
Obinutuzumab is an Fc-engineered type II anti-CD20 monoclonal antibody.23 Much like rituximab, obinutuzu-mab targets the CD20 antigen expressed on B-lymphocytes and activates complement-dependent cytotoxicity.23 Obinutuzumab differs from rituximab by its design; it binds to a different epitope on the CD20 antigen and leads to enhanced immune effector functions, especially antibody-dependent cell-mediated cytotoxicity.23
Although type II monoclonal antibodies bind more weakly to complement proteins and have decreased complement-dependent cytotoxicity activity, direct apoptosis is increased.24 The Fc region in antibodies interacts with cell-surface receptors, resulting in activation of the immune system and an increased opsonization, cell lysis, and other processes.24 Obinutuzumab produces greater B-cell depletion compared with rituximab.23
A recent study by Reslan and colleagues showed that obinutuzumab had a significantly greater effect on apoptosis compared with rituximab.25 Furthermore, in preclinical studies, obinutuzumab demonstrated superiority over rituximab in isolated CLL cells.26
Obinutuzumab Pharmacokinetics
The elimination of obinutuzumab comprises a linear clearance pathway and a time-dependent nonlinear clearance pathway. The half-life elimination of obinutuzumab is approximately 28.4 days and the volume of distribution is 3.8 L.27 The pharmacokinetics of this drug is not affected by a patient’s age.27 The pharmacokinetics of obinutuzumab was studied in a phase 1 clinical trial.28 A total of 14 days after induction therapy with obinutuzumab, the plasma concentrations of obinutuz-umab remained elevated with doses ≥1000 mg. Rapid decline of serum drug concentrations was observed with doses <1000 mg. To maintain saturation of the target (CD20), obinutuzumab doses of at least 1000 mg were required.28 Based on these results, a maintenance dose of 1000 mg was chosen for further clinical studies.
Obinutuzumab Clinical Efficacy
Phase 2 Clinical Trials
A phase 2 clinical trial investigated the dose-response relationship of obinutuzumab in patients with relapsed or refractory non-Hodgkin lymphoma.29 A total of 40 patients were enrolled in the study: 38 of them had received previous rituximab therapy. In all, 18 patients were randomly assigned to receive an initial dose of obinutuzumab of 400 mg and a maintenance dose of 400 mg, and 22 patients were assigned to receive 1600 mg of obinutuzumab and a maintenance dose of 800 mg. The overall response rate was 55% in the 1600-mg/800-mg treatment arm, with 9% complete responders. In the 400-mg/400-mg treatment arm, the overall response rate was 17%, with no complete responders. The median progression-free survival (PFS) was 11.9 months in the higher-dosed group and 6 months in the lower-dosed group.29 This study showed promising results, but because of the small sample size, the study was not statistically powered to assess the significant differences between the 2 groups. Most adverse events were infusion-related reactions. No patients withdrew because of adverse events.29
Another phase 2 clinical trial was conducted to determine the clinical efficacy of obinutuzumab in patients with diffuse large B-cell lymphoma or mantle-cell lymphoma who were heavily pretreated.30 A total of 40 patients were enrolled. Of these, 21 patients were randomly assigned to receive an initial dose of 400 mg and a maintenance dose of 400 mg, and 19 patients were randomly assigned to receive an initial dose of 1600 mg and a maintenance dose of 800 mg. The results showed no significant difference in PFS between the 2 treatment arms. Furthermore, the larger dose had a better overall response rate than the flat dose of 400 mg, because the steady-state serum concentration was reached. This study confirmed that a higher dose of obinutuzumab is needed to elicit a response in patients with lymphoma.30
Pivotal Phase 3 Clinical Trial
Based on encouraging results from the phase 2 clinical trials, a phase 3, multicenter, international 3-arm protocol was initiated to compare obinutuzumab combined with chlorambucil; rituximab combined with chlorambucil; or chlorambucil monotherapy in treatment-naïve patients with CLL and with coexisting conditions.31
This study included 781 patients who were randomized to 3 treatment arms.31 In all 3 treatment arms, chlorambucil was administered orally at a dose of 0.5 mg/kg. The first treatment arm included obinutuzumab, which was administered intravenously at a dose of 1000 mg on days 1, 8, and 15 of cycle 1 and on day 1 of cycles 2 through 6, plus chlorambucil, which was administered on days 1 and 15 of cycles 1 through 6. The second treatment arm included rituximab, which was administered intravenously at a dose of 375 mg/m2 on day 1 of cycle 1, and 500 mg/m2 on day 1 of cycles 2 through 6, plus chlorambucil. The third treatment arm included chlorambucil alone.31
The primary outcome measure for this study was investigator-assessed PFS.31 Treatment lasted for more than 6 months, and the follow-up for disease-progression and safety is for at least 5 years.32 Efficacy results, including the PFS, response rate, and hazard ratio (HR) among the treatment arms are summarized in Table 1.31
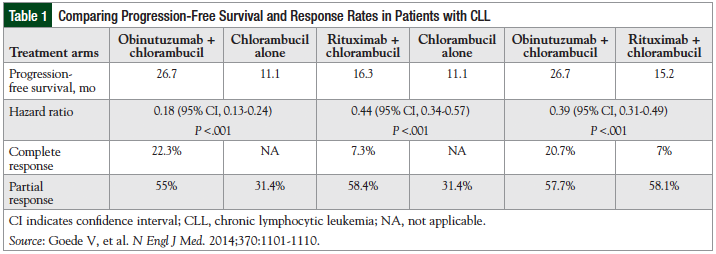
The group of patients (N = 238) receiving obinutuz-umab plus chlorambucil achieved a median PFS of 26.7 months compared with 11.1 months with chlorambucil alone (N = 118; P <.001), for an increase of 15.6 months with obinutuzumab plus chlorambucil.31 The overall response rate was 77.3% for the combination therapy group and 31.4% for the chlorambucil-alone group.31 In addition, the HR for progression or death between therapy with obinutuzumab plus chlorambucil compared with chlorambucil alone is 0.18, which translates to an 82% risk reduction in patients who are receiving obinutuz-umab plus chlorambucil versus chlorambucil alone.27
The PFS with obinutuzumab plus chlorambucil was also significantly better than the PFS with rituximab plus chlorambucil (26.7 months vs 15.2 months, respectively; P <.001).31 In addition, the overall survival with obinutuz-umab plus chlorambucil compared with chlorambucil alone was significantly better, with a HR of 0.41 (95% confidence interval [CI], 0.23-0.74; P = .002). There was no significant difference between the overall survival with obinutuzumab plus chlorambucil and rituximab plus chlorambucil (HR, 0.66; 95% CI, 0.41-1.06; P = .08]).31
This pivotal trial led to the FDA approval of obinutuzumab plus chlorambucil for the treatment of patients with CLL.22
Dosing
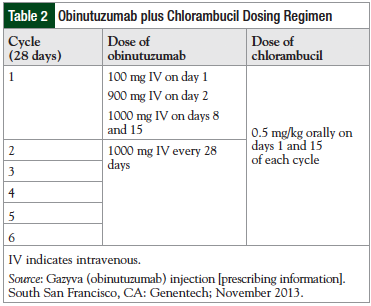
The prescribing product information describes the recommended dosing regimen for obinutuzumab plus chlorambucil for the treatment of patients with previously untreated CLL (Table 2).27
Safety
Infusion-Related Reactions
Studies performed in non-Hodgkin lymphoma and B-cell malignancies suggest that obinutuzumab is well-tolerated.27,33 The primary adverse events reported with obinutuzumab, which is administered intravenously, have been infusion-related reactions. The adverse events reported in the phase 3 trial are listed in Table 3.31
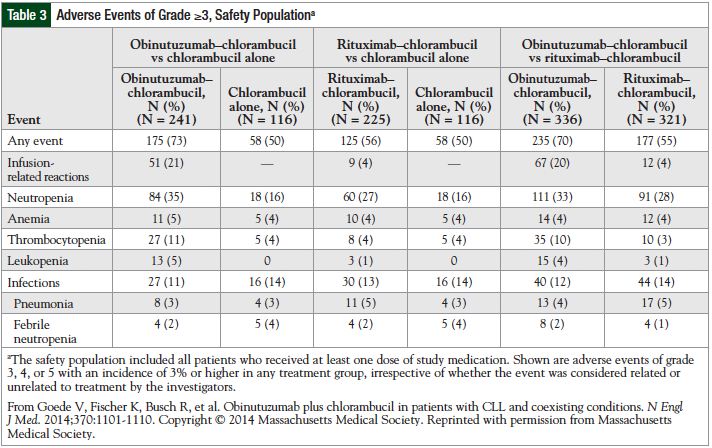
Infusion-related reactions reported include hypotension, pyrexia, nausea, vomiting, chills, asthenia, flushing, headache, and larynx irritation. Most reactions were observed during the first infusion. No patients discontinued the treatment because of toxicities. Infusion-related reactions resolved after slowing or interrupting the infusion. Adverse events that required hospitalization included anemia, neutropenia, thrombocytopenia, and tumor lysis.33
Although a humanized antibody, obinutuzumab produced a substantial number of infusion-related reactions in phase 1 and phase 2 trials: 55% to 83% of infusion-related reactions were grade 1 or 2, and 8% to 18% of infusion-related reactions were grades 3 or 4.34 Furthermore, in the phase 3 study by Goede and colleagues, infusion-related reactions were more common in the obinutuzumab-plus-chlorambucil group than in the chlorambucil-alone group.31 Reactions may be related to many factors, including drug concentration, pH of the solution, infusion rate of the drug, or diluents.27
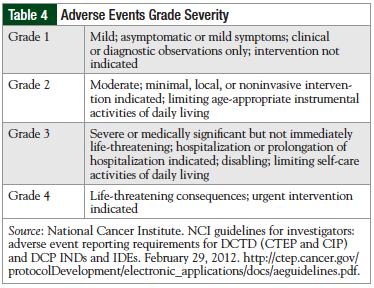
For patients who develop infusion-related reactions rated grade 1 or grade 2, a reduced infusion rate or interruption is recommended to manage symptoms.27 For grade 3 reactions, an interruption in therapy is recommended. Patients who develop a grade 4 reaction are recommended to discontinue immediately and permanently.27 The degrees of severity of adverse events are defined in Table 4.35
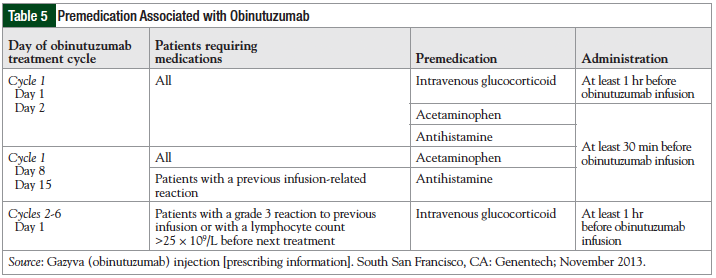
Premedication is often required before the administration of monoclonal antibodies to reduce the risk of infusion-related reactions.27 Premedication includes antihistamines, glucocorticoids, and acetaminophen. The premedication regimen for obinutuzumab infusion is listed in Table 5.27
Boxed Warnings
Obinutuzumab contains a boxed warning with 2 warnings, which include hepatitis B virus (HBV) reactivation and progressive multifocal leukoencephalopathy (PML).
Screening for HBV before treatment is required.27 Patients with high levels of hepatitis B may be monitored with serial measurements of hepatitis B viral load and treated if the viral load increases. If treatment is started in patients with HBV infection, prophylactic antiviral therapy is recommended. Patients with prophylactic treatment need to be monitored for hepatitis B viral load monthly during, and every 3 months after, treatment. The NCCN panel recommended that prophylactic treatment is to be maintained for up to 12 months after treatment with obinutuzumab ends.4,27
The diagnosis of PML should be considered in any patient presenting with new onset or changes to preexisting neurologic indicators. In patients who develop PML, obinutuzumab therapy should be discontinued.
Use in Specific Populations
Obinutuzumab is classified as pregnancy category C. There are no adequate and well-controlled studies of obinutuzumab in pregnant women. Animal studies have shown that there were no teratogenic effects. Pediatric use has not been established because CLL is typically a disease of the elderly. For geriatric use, there were no significant differences in efficacy between patients aged ≥75 years and patients aged <75 years. In patients who have renal or hepatic impairment, no dosage adjustments have been studied or provided in the manufacturer’s labeling.27
Cost Considerations
The average wholesale price of obinutuzumab 1000 mg in a 40-mL intravenous solution is estimated to be $6192.36 A full course of obinutuzumab therapy is 8000 mg, which would amount to approximately $49,536 (depending on the purchase by an institution).
Place in Therapy
In the phase 3 trial that led to its approval, obinutuz-umab in combination with chlorambucil demonstrated a significant increase in PFS benefit, overall response rate, and complete response rate compared with chlorambucil alone.31 Based on this evidence, the FDA fast tracked it through the review process for the treatment of patients with CLL. The current NCCN guidelines for CLL include obinutuzumab plus chlorambucil as the preferred initial treatment for patients with CLL without 11q or 17p deletions and as the preferred initial treatment for patients with 11q deletion. For patients with deletion 17p, obinutuzumab plus chlorambucil is a first-line therapy choice.5
Conclusion
Obinutuzumab, an Fc-engineered type II anti-CD20 monoclonal antibody, has been shown to be effective in the treatment of patients with CLL, and is the first cancer drug with a breakthrough therapy designation to receive FDA approval. Obinutuzumab in combination with chlorambucil is now the preferred first-line regimen for patients aged ≥70 years with CLL that requires pharmacotherapy. It is also the preferred first-line regimen for any patient with CLL and deletion 11q.
Author Disclosure Statement
Mr Tang, Dr Lindfelt, and Dr Ignoffo have no conflicts of interest to report.
References
- American Cancer Society. What are the key statistics for chronic lymphocytic leukemia? Updated April 18, 2014. www.cancer.org/cancer/leukemia-chroniclymphocyticcll/
detailedguide/leukemia-chronic-lymphocytic-key-statistics. Accessed May 27, 2014. - Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456. Erratum in: Blood. 2008;112:5259.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): non-Hodgkin’s lymphomas. Version 2.2014. March 27, 2014. www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed May 29, 2014.
- Parker TL, Strout MP. Chronic lymphocytic leukemia: prognostic factors and impact on treatment. Discov Med. 2011;11:115-123.
- Thomas R, Ribeiro I, Shepherd P, et al. Spontaneous clinical regression in chronic lymphocytic leukaemia. Br J Haematol. 2002;116:341-345.
- Rai KR, Keating MJ. Staging and prognosis of chronic lymphocytic leukemia. UpToDate. www.uptodate.com/contents/staging-and-prognosis-of-chronic-lymphocytic-leukemia. Updated April 16, 2014. Accessed May 29, 2014.
- American Cancer Society. Cancer facts and figures 2013. www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. Accessed December 13, 2013.
- Smolewski P, Witkowska M, Korycka-Wołowiec A. New insights into biology, prognostic factors, and current therapeutic strategies in chronic lymphocytic leukemia. ISRN Oncol. 2013;2013:740615.
- US Food and Drug Administration. FDA approves rituxan to treat chronic lymphocytic leukemia. Press release. February 18, 2010. Updated April 25, 2013. www.fda.gov/newsevents/newsroom/pressannouncements/ucm201069.htm. Accessed May 27, 2014.
- Demko S, Summers J, Keegan P, Pazdur R. FDA drug approval summary: alemtuz-umab as single-agent treatment for B-cell chronic lymphocytic leukemia. Oncologist. 2008;13:167-174.
- US Food and Drug Administration. FDA approves new treatment for chronic lymphocytic leukemia. Press release. October 26, 2009. Updated April 17, 2014. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187966.htm. Accessed May 27, 2014.
- US Food and Drug Administration. Ofatumumab. April 17, 2014. Updated April 17, 2014. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm393823.htm. Accessed May 27, 2014.
- Kipps TJ. Chronic lymphocytic leukemia and related diseases. In: Kaushansky K, Lichtman MA, Beutler E, et al, eds. Williams Hematology. 8th ed. New York, NY: McGraw-Hill; 2010:1431-1482.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): non-Hodgkin’s lymphomas. Version 2.2013. September 6, 2013. www.oncomap.org/download_zhinan/%E6%8C%87%E5%8D%97/nhl.pdf. Accessed November 26, 2013.
- Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910-1916.
- Neilson JR, Auer R, White D, et al. Deletions at 11q identify a subset of patients with typical CLL who show consistent disease progression and reduced survival. Leukemia. 1997;11:1929-1932.
- Hallek M. Signaling the end of chronic lymphocytic leukemia: new frontline treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013:138-150.
- Tsai PC, Hernandez-Ilizaliturri FJ, Bangia N, Olejniczak SH, Czuczman MS. Regulation of CD20 in rituximab-resistant cell lines and B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18:1039-1050.
- Jaglowski SM, Alinari L, Lapalombella R, Muthusamy N, Byrd JC. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood. 2010;
116:3705-3714. - US Food and Drug Administration. Regulatory information: frequently asked questions: breakthrough therapies. Updated May 22, 2014. www.fda.gov/regula
toryinformation/legislation/federalfooddrugandcosmeticactfdcact/significantamendmentstothefdcact/fdasia/ucm341027.htm. Accessed May 29, 2014. - US Food and Drug Administration. FDA approves Gazyva for chronic lymphocytic leukemia. Press release. November 1, 2013. Updated November 1, 2013. www.fda.gov/newsevents/newsroom/pressannouncements/ucm373209.htm. Accessed May 27, 2014.
- Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393-4402.
- Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823-3837.
- Reslan L, Dalle S, Herveau S, et al. Apoptotic induction by anti-CD20 antibodies in chronic lymphocytic leukemia: comparison of rituximab and obinutuzumab. Leuk Lymphoma. 2014;55:188-190.
- Patz M, Isaeva P, Forcob N, et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol. 2011;152:295-306.
- Gazyva (obinutuzumab) injection [prescribing information]. South San Francisco, CA: Genentech; November 2013.
- Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119:5118-5125.
- Salles GA, Morschhauser F, Solal-Céligny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2920-2926.
- Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2912-2919.
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101-1110.
- CLL11: A Study of Obinutuzumab (RO5072759 [GA101]) With Chlorambucil in Patients With Previously Untreated Chronic Lymphocytic Leukemia (Stage 1a). Trial NCT01010061. http://clinicaltrials.gov/show/NCT01010061. Accessed June 5, 2014.
- Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119:5126-5132.
- Mancheril BG, Aubrey Waddell J, Solimando DA Jr. Drug monographs: afatinib and obinutuzumab. Hosp Pharm. 2014;49:237-241.
- National Cancer Institute. NCI guidelines for investigators: adverse event reporting requirements for DCTD (CTEP and CIP) and DCP INDs and IDEs. February 29, 2012. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/aeguidelines.pdf. Accessed May 19, 2014.
- Lexi-Drugs Online. Obinutuzumab. Hudson, OH: Lexi-Comp, Inc. http://0-online.lexi.com.library.touro.edu/lco/action/doc/retrieve/docidpatch_f/4801839?hl=700830. Accessed February 6, 2014.
