The Multinational Association of Supportive Care in Cancer, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network (NCCN) each publish antiemetic and supportive care guidelines for patients who are undergoing chemotherapy treatment.1-3 These guidelines serve as resources for evidence-based antiemetic decisions. Antiemetic therapy is based on the emetogenic potential of the chemotherapy agents used in the regimen.
For single-day highly emetogenic chemotherapy regimens (eg, high-dose cisplatin, high-dose doxorubicin), a 5-hydroxytryptamine (5-HT3) receptor antagonist, steroid, and neurokinin 1 (NK1) receptor antagonist are recommended on day 1 of chemotherapy use. In addition, steroids are recommended on day 2 through day 4 of the regimen.3 If the NK1 receptor antagonist chosen on day 1 is oral aprepitant, continuation of aprepitant through day 3 is recommended. Patients who receive intravenous (IV) fosaprepitant on day 1 should not receive an NK1 receptor antagonist on days 2 and 3.3
When administering a single-day, moderately emetogenic chemotherapy regimen (eg, IV melphalan or cyclophosphamide ≤1500 mg/m2), a 5-HT3 receptor antagonist and a steroid (with or without an NK1 receptor antagonist) should be administered on day 1 of initiating chemotherapy.3 On day 2 and day 3, monotherapy with a 5-HT3 receptor antagonist or a steroid is recommended. If an NK1 receptor antagonist is used on day 1, its use should continue on days 2 and 3 only if oral aprepitant is the selected therapy.3
When administering multiday chemotherapy regimens, it is difficult to recommend a specific antiemetic regimen for each day, especially because acute and delayed emesis may overlap after the initial day of chemotherapy through the last day of chemotherapy.3 With multiday regimens, dexamethasone (oral or IV) should be administered once daily on the days of chemotherapy and for 2 to 3 days after chemotherapy with regimens that are likely to cause significant delayed emesis. In addition, a serotonin antagonist should be administered before the first dose and the subsequent doses of moderately or highly emetogenic chemotherapy.3
For the treatment of breakthrough nausea and vomiting, as-needed (PRN) antiemetic medications should be available. Ideally, these medications would act via a different mechanism from those of the scheduled antiemetic regimens. If the PRN medication is effective for the patient, it should be scheduled and considered in future chemotherapy cycles.3 If the PRN medication is ineffective, a different dose, or a different antiemetic medication, should be considered. When a patient experiences substantial nausea and vomiting despite the scheduled antiemetic medications, increasing to a regimen with a higher emetogenicity profile may be warranted (ie, using the highly emetogenic regimen for a patient who did not respond to the moderately emetogenic antiemetic regimen).2
Within the Allina Health system, standardized paper chemotherapy order sets are used for medication ordering and prescribing. Before the standardization of antiemetic medications, antiemetic agents were ordered and prescribed inconsistently for patients receiving chemotherapy. In addition, the antiemetic regimens and agents used were not stratified based on the emetogenic potential of the chemotherapy regimen.
The study was based on the assumption that optimizing the prescribing of antiemetic medications may reduce the potential for nausea and vomiting events associated with chemotherapy. The purpose of this study was therefore to evaluate the impact of standardizing antiemetic medications on select chemotherapy order sets within a large healthcare system, such as Allina Health. The elements evaluated include (1) the appropriateness of antiemetic therapy compared with the NCCN guideline recommendations, (2) the number of nausea complaints, (3) the frequency of PRN antiemetic use, and (4) the number of vomiting events in the prestandardization group (ie, prestandardized order sets) and in the poststandardization group (ie, poststandardized order sets). This study also sought to identify potential barriers and limitations to provider’s compliance with guideline-based ordering of antiemetic medications.
Methods
For this study, inpatient chemotherapy regimens were stratified as highly emetogenic or moderately emetogenic using the NCCN antiemetic guidelines, version 1.2012.3 A group of oncology pharmacists created universal antiemetic order sets—1 antiemetic order set for highly emetogenic regimens and 1 antiemetic order set for moderately emetogenic regimens––based on the NCCN clinical practice guidelines.3 Standardized antiemetics were integrated into select order sets on March 5, 2013. The highly emetogenic order sets included DHAP (dexamethasone, high-dose cytarabine, and cisplatin); CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), with or without rituximab; and cisplatin plus lomustine.
The moderately emetogenic order sets included ICE (ifosfamide, carboplatin, and etoposide) for lymphoma; CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), course A and B; and the acute myeloid leukemia (AML) induction regimen known as “7 + 3.”
The health system’s electronic medical records (EMRs) were utilized to identify patients for study inclusion who received inpatient chemotherapy from the predetermined order sets between November 2011 and December 2012. Data were retrospectively collected from 3 of the metro area hospitals within this health system. The EMRs of a randomly selected sample of 50 patient encounters between November 2011 and December 2012 were included in the prestandardization group. A total of 32 patient encounters between March 5, 2013, and June 6, 2013, were included in the poststandardization group.
The patient encounters were compared in the pre- and poststandardization periods to identify whether a decreased use of PRN antiemetics, decreased incidence of nausea complaints, and/or decreased incidence of vomiting occurred as a reflection of standardizing the antiemetic therapy. Nausea complaints and vomiting events were evaluated based on nursing documentation in the EMRs. This study involved retrospective data extraction, and it was exempt from review by the Allina Institutional Review Board.
Results
The treatment regimens of the 50 patient encounters in the prestandardization group and the 32 patient encounters in the poststandardization group were compared. Table 1 outlines the patient distribution of the treatment regimens for the pre- and poststandardization phases of the study.
The most frequently administered regimen in the prestandardization group was the AML induction regimen (categorized as moderately emetogenic, days 1-3), with 15 of the 50 patients receiving this therapy. The most frequently administered regimen in the poststandardization group was hyper-CVAD, course A and B (categorized as moderately emetogenic), with 12 of the 32 patients receiving this regimen.
The baseline patient characteristics (ie, age and sex distribution) were similar between the 2 groups (Table 2).
Overall, patients whose antiemetic agents were ordered and prescribed from the poststandardized order sets required fewer PRN medications and complained of nausea less frequently than patients in the prestandardization group (Table 2). The average use of PRN medications for breakthrough nausea and vomiting was 12.8 events (range, 0-115) in the prestandardization group compared with 5.9 events (range, 0-44) in the poststandardization group.
In addition, the average number of nausea complaints as documented in the EMR system was 2.2 events (range, 0-22) in the poststandardization group compared with 5 events (range, 0-45) in the prestandardization group. The average number of vomiting events was 1.03 in the poststandardization group compared with 0.54 in the prestandardization group. This was thought to be the result of 1 specific patient; this patient stayed in the hospital for 34 days, and the nausea and vomiting did not occur until well after chemotherapy had been completed (ie, day 10 of chemotherapy).
Excluding this patient, vomiting events averaged 0.47 per patient hospital stay in the poststandardization group. This is slightly less than 0.54 events in the prestandardization group.
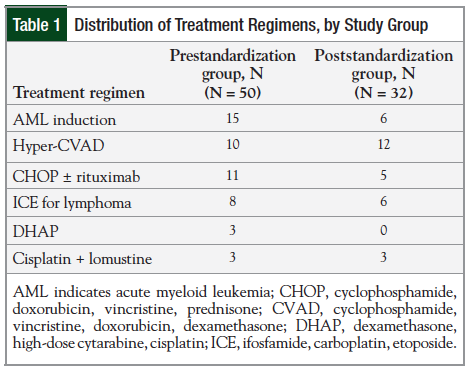
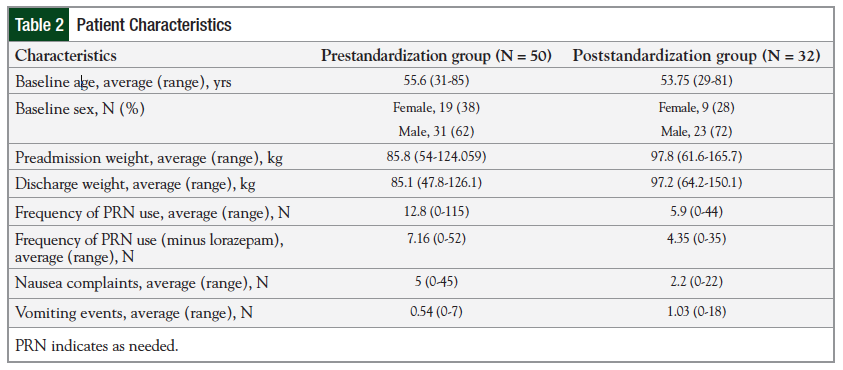
The results for the moderately emetogenic regimens are presented in Figure 1 and Figure 2.
The prescribing of NK1 receptor antagonists increased from 3% in the prestandardization group to 40% in the poststandardization group. The use of NK1 receptor antagonists is optional for moderately emetogenic chemotherapy, according to the NCCN guidelines.3 In addition, the prescribing of PRN medications with different mechanisms of action increased from 69% (range, 65.6%-72.7%) in the prestandardization group to 88% in the poststandardization group.
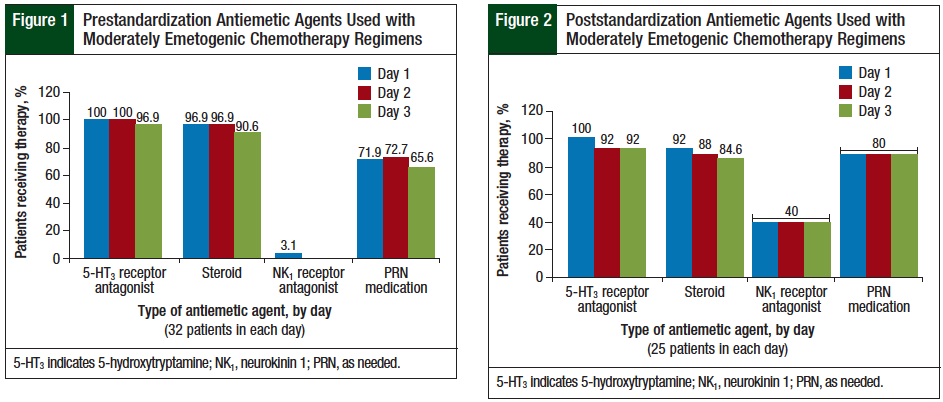
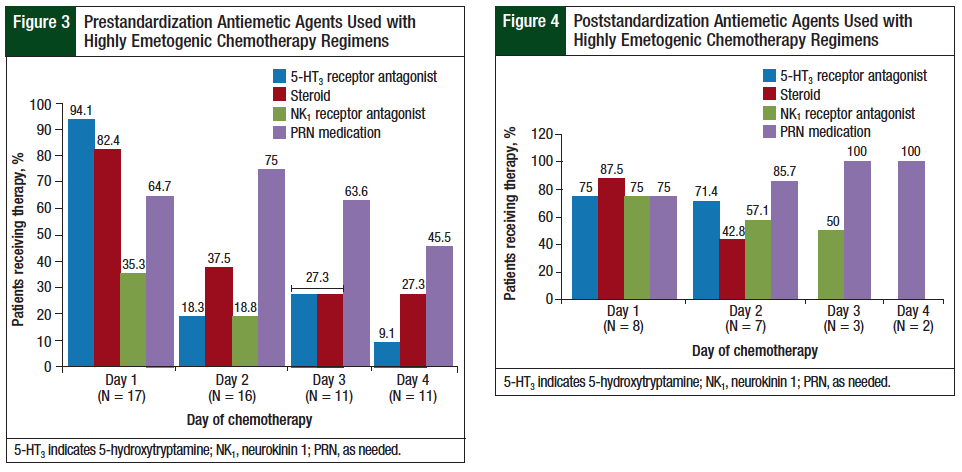
The NCCN guidelines recommend the use of a 5-HT3 receptor antagonist and a steroid on every day of a highly emetogenic chemotherapy regimen and an oral NK1 receptor antagonist on day 1 through day 3, or an NK1 receptor antagonist administered intravenously on day 1.3 The results for the highly emetogenic regimens in this study are presented in Figure 3 and Figure 4.
The use of scheduled 5-HT3 receptor antagonists on day 1 decreased from 94% in the prestandardization group to 75% in the poststandardization group. In this study, steroid prescribing for highly emetogenic regimens on day 1 and day 2 was similar between the prestandardization and the poststandardization groups. The use of NK1 receptor antagonists on day 1 of highly emetogenic regimens increased from 35% in the prestandardization group to 75% in the poststandardization group. The prescribing of PRN medications with different mechanisms of action increased from 60% (range, 45.5%-75%) in the prestandardization group to 87% (range, 75%-100%) in the poststandardization group.
Discussion
The NCCN clinical practice guidelines were chosen as the main resource for order set standardization, because these guidelines are updated annually and are consensus based. Overall, the establishment of antiemetic medication standardization of chemotherapy order sets at Allina Health reduced the average number of nausea complaints and the need for PRN antiemetic medications during inpatient chemotherapy days.
In addition, there was a marked improvement in the use of NK1 receptor antagonists during the administration of highly emetogenic chemotherapy regimens. Furthermore, the prescribing of PRN breakthrough medications improved in patients receiving moderately emetogenic and highly emetogenic chemotherapy regimens. Although breakthrough nausea and vomiting are often difficult to manage and control, it is important for antiemetic medications to be available, should the need arise.
There are still opportunities for improvement and reeducation of the standardized antiemetic order sets. After standardization of the antiemetic agents on the order sets, there was a slight decrease in the use of 5-HT3 receptor antagonists on day 1 of administering the highly emetogenic regimens. Because nausea and vomiting are often very individualized, achieving 100% adherence with prescribing based on the NCCN guidelines is unlikely.
This study did not identify any barriers to adherence to the order sets or the order set standardization. Before the standardized order sets were released, the oncology providers in this study were educated on the change. The providers were encouraged to use the standardized antiemetic agents on the order sets.
Limitations
This analysis has several limitations. Because the study includes data from multiple hospital sites within the Allina Health system, one potential limitation is that the sample groups are not evenly matched.
In addition, the increased number of vomiting events seen in the poststandardization group was largely attributed to a single patient who experienced substantial nausea and vomiting. Furthermore, the sample size for patient encounters for patients who received highly emetogenic chemotherapy regimens in the poststandardization phase was small, with only 8 patients. Excluding this patient would reduce the rate of vomiting events poststandardization.
Conclusion
The results of this study suggest that standardizing antiemetic medication use on oncology order sets based on the emetogenic potential of the chemotherapy regimen used can reduce the use of PRN medications and decrease the number of nausea events associated with the administration of chemotherapy. These changes can provide benefits to patients with cancer and improve quality of life. The results of this study further indicate that monitoring order set adherence and updating chemotherapy order sets periodically based on changes in treatment guidelines is imperative. It is important to provide patients with effective, evidence-based antiemetic therapy to control the nausea and vomiting associated with chemotherapy.
Author Disclosure Statement
Dr Zarambo, Dr Brenner, and Ms Sather reported no conflicts of interest.
References
- Roila F, Herrstedt J, Aapro M, et al; for the ESMO/MASCC Guidelines Working Group. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232-v243.
- Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189-4198.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™): antiemesis. Version 1.2012. July 20, 2011. http://www5.medicine.wisc.edu/~williams/antiemesis.pdf. Accessed March 3, 2013.