The increased use of oral chemotherapy presents many challenges to patients and providers, including high medication costs, complex regimens, and lack of care coordination. In addition, because patients ingest these agents outside of the clinic, healthcare providers cannot directly monitor for compliance and patient safety. Therefore, proper communication and patient education are essential to increasing the safe use of oral chemotherapy.1-5
The American Society of Clinical Oncology (ASCO) and the Oncology Nursing Society (ONS) revised their chemotherapy standards in 2013 to include statements regarding the management of oral chemotherapy. In the guidelines, the authors recommend that specific components be included on all prescriptions for oral chemotherapy.6 These components, such as reference to dose calculation, provide additional information to ensure that medications are dosed appropriately. Many of the components are consistent with nonoral chemotherapy prescriptions, including name and second identifier, drug name, date, dosage (without trailing zeros and including leading zeros), quantity, frequency, route of administration, and refills (including 0 if no refills are prescribed). The ASCO/ONS guidelines, however, also require more specific information to verify that prescriptions are written correctly; specifically, the prescription should contain a reference to how the dose is calculated. The patient's height and weight should be included if that information is needed to calculate the dose. If the prescription is noncontinuous, the duration of therapy should also be listed in the directions. Although pharmacists were not involved in the development of these guidelines, studies have shown that pharmacist participation in the ordering and processing of oral chemotherapy increases the accuracy of prescribing.7 By assisting with coordination of care (eg, aiding with patient assistance or prior authorizations), pharmacists could decrease delays to starting oral chemotherapy treatment.8
We conducted a retrospective cohort study to evaluate the impact of pharmacists on increasing prescriber adherence to the 2013 ASCO/ONS guidelines, patient safety, and time-to-treatment initiation. Revenue generated at the on-site retail pharmacy was also assessed.
Methods
This study was an Institutional Review Board–approved, retrospective chart review of the electronic medical record (EMR) and prescription records to examine the effects of a pharmacist-driven oral chemotherapy (PDOC) program at an outpatient National Cancer Institute (NCI)-designated cancer center associated with an academic medical center.
As part of an NCI-designated cancer center affiliated with an academic medical center, we developed a PDOC to increase patient safety. The clinical pharmacists embedded within the outpatient neuro-oncology and gastrointestinal (GI) oncology clinics verified order accuracy, medication procurement, and patient education associated with oral chemotherapy. The decision to initiate the program within these clinics was made because capecitabine and temozolomide require a dose calculation for prescribing that increases the likelihood for medication errors.9
The workflow for the PDOC is described in Figure 1. Although electronic prescribing was available, we first developed preprinted order sets for oral chemotherapy prescriptions, including agents such as tyrosine kinase inhibitors (Figures 2 and 3; Supplemental Figure online). The preprinted order sets contained references to the specific chemotherapy regimen, and fields for laboratory and monitoring parameters. They were implemented because the EMR at the time of the study was not robust enough to transmit all of the data needed for safe and accurate prescribing of oral chemotherapy. Prescribers used the order forms to write oral chemotherapy prescriptions, then an in-clinic pharmacist reviewed each prescription.
After the first pharmacist check, the preprinted order forms were scanned into the EMR, where a second pharmacist, either in the on-site retail pharmacy or another outpatient clinic, reviewed the order for accuracy. For every prescription, 2 pharmacists independently verified each prescription for accuracy and drug interactions, regardless of where the prescription was ultimately filled. Pharmacists embedded within the clinic setting also help with patient counseling, medication procurement, and provide a follow-up phone call within 3 days of the patient beginning oral chemotherapy. During this phone call, the pharmacist ensured that the patient was taking the medication as prescribed, and monitored for adverse events or side effects. After this initial assessment, the pharmacist determined which patients needed further follow-up, and contacted those patients at a later date. The pharmacist's follow-up phone call, benefit assessment, and counseling were not routinely completed for prescriptions from the non-PDOC clinics.
Inclusion Criteria
To be included in this study, patients had to be aged ≥18 years and treated in one of the solid-tumor outpatient oncology clinics. Patients had to receive a new start oral chemotherapy prescription between April 1, 2013, and August 31, 2013. New-start oral chemotherapy was defined as a regimen being received by the patient for the first time; this could not be a refilled prescription.
Refill prescriptions, or a new prescription that was a continuation of a prior regimen, were not included in the study. These prescriptions were excluded because the pharmacist follow-up phone call was not standard for all refills. Patients were identified through clinic reports generated by the EMR for each oncology provider. In addition, outpatient prescription records from the on-site retail pharmacy were reviewed to further identify patients with an oral chemotherapy prescription.
Outcomes
The primary outcome was to determine adherence to the 2013 ASCO/ONS prescription standards for oral chemotherapy. To assess this outcome, oral chemotherapy orders and prescriptions were reviewed to determine whether they included the components specified in the ASCO/ONS guidelines.6 Prescriptions from the PDOC (neuro-oncology and GI oncology clinics) were compared with prescriptions from the clinics without an oncology pharmacist (non-PDOC). Prescriptions from the non-PDOC group were sent electronically from the clinic to the retail pharmacy without an internal pharmacist review. If the prescription was sent to the on-site retail pharmacy, it was screened by the retail pharmacist. However, this review process was not standardized among the non-PDOC group, and differed from the review provided by the PDOC.
Secondary outcomes included describing safety interventions made by the pharmacist during initial review or follow-up phone call, comparing time-to-treatment initiation between groups (PDOC vs non-PDOC), and quantifying revenue generated in the associated outpatient retail pharmacy. To assess safety interventions, all documentation made by the pharmacists in the EMR or in a data collection folder was reviewed. Interventions were classified into the following categories: completion of orders, dose adjustments, ensuring that patients received medication calendars for noncontinuous regimens, providing patient education, managing side effects at the time of pharmacist follow-up, assisting with medication procurement, adjusting for drug allergies, and managing drug interactions.
A pharmacist not involved in the PDOC or data collection reviewed the interventions and rated their significance based on a previously tested scale,10 which rates the potential severity of the error prevented, and the clinical value of service. This scale has been tested by pharmacists and physicians as a reliable way to classify pharmacist interventions.10 In this study, pharmacist interventions from the PDOC were ranked based on their clinical significance. If the intervention involved correcting an error, it was further classified by the severity of the error.
Time-to-treatment initiation was compared between the 2 groups to determine whether pharmacist intervention decreased treatment delays. Prescriptions were considered to be filled on time if they were filled on the same day as prescribed or before the designated start date (if this was documented in the chart). To determine time-to-treatment initiation, patient EMRs and pharmacist interventions were reviewed for documentation of expected and actual start dates. Prescription records from the on-site retail pharmacy were reviewed as well to determine if these prescriptions were filled on time.
Revenue from oral chemotherapy and associated supportive care medications filled on the same day were compared with revenue missed by patients who filled their prescription at external pharmacies. For prescripions filled at the on-site retail pharmacy, revenue was determined by subtracting the cost of the medication from the payments received, as documented in the retail prescription profile. For presriptions eligible to be filled via Medicare Part B benefits, the revenue was estimated using 80% of the maximum allowable charge for each medication–because Medicare pays this portion–and by subtracting the patient copay. This estimation was used because during the study period, patients did not pay their out-of-pocket requirement at the time of adjudication. Therefore, this method provided a conservative estimate on revenue generation. To estimate missed revenue opportunities, prescriptions filled outside of the associated retail pharmacy were matched with controls that had similar reimbursement plans and known costs.
Statistical Analysis
Descriptive statistics were used to assess all data. Fisher's exact test was used to determine statistical differences for categorical data.
Results
Patients
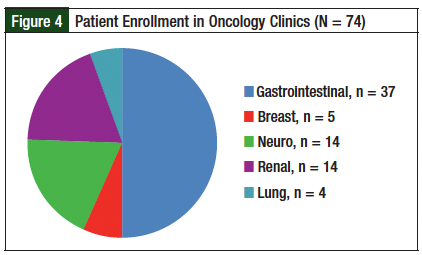
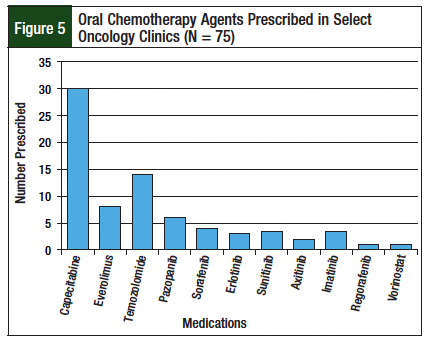
Seventy-four patients and 75 new-start prescriptions were included in the study; 1 patient had 2 prescriptions for oral chemotherapy during the evaluation period. Of these prescriptions, 68% (n = 51/75) were from clinics with the PDOC (neuro-oncology and GI oncology), and 32% (n = 24/75) were from the non-PDOC clinics (breast, renal, and lung). Figures 4 and 5 describe patient enrollment in specific clinics and medications prescribed, respectively.
Compliance with 2013 ASCO/ONS Requirements
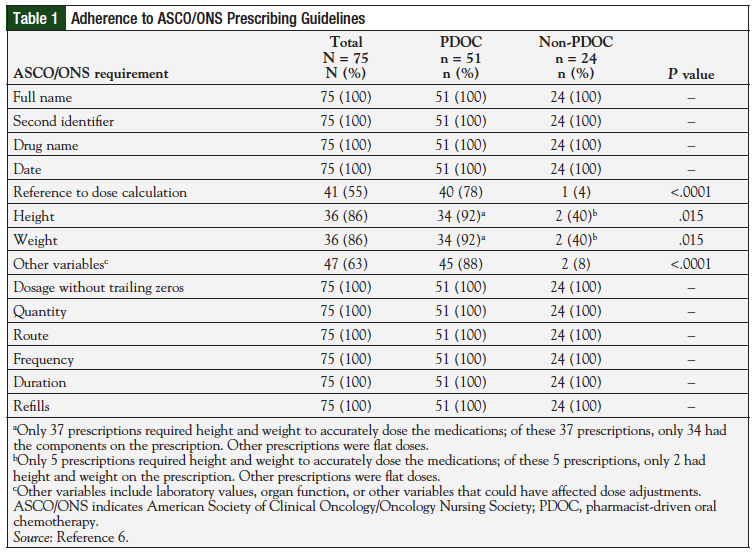
Table 1 describes the compliance with the 2013 ASCO/ONS chemotherapy standards in each group.6 Seventy-eight percent (n = 40/51) of prescriptions associated with the PDOC had all the required 2013 ASCO/ONS components. In contrast, none (n = 0/24) of the non-PDOC prescriptions had all the required 2013 ASCO/ONS components. The PDOC group contained reference to dose calculation in 78% (n = 40/51), height and weight–when required to accurately dose the medication in 92% (n = 34/37), and other relevant information (eg, laboratory information, organ function) in 88% (n = 45/51) of prescriptions. The missing components for these prescriptions were not located on the prescriptions, or on the preprinted order sets. Of all prescriptions in the non-PDOC group, 4% (n = 1/24) included reference to dose calculation, 40% (n = 2/5) included height and weight when required, and 8% (n = 2/24) included other relevant information (eg, laboratory information, organ function). All prescriptions from both clinics contained the remaining 2013 ASCO/ONS components. Overall, pharmacist intervention statistically increased compliance with reference to dose calculation (P <.0001), height and weight (P = .015), and other pertinent information (P <.0001) compared with the prescriptions without pharmacist review.
Safety Interventions
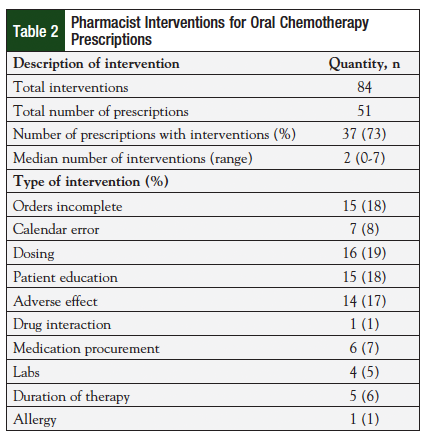
Pharmacists made a total of 84 interventions for 51 prescriptions, with a median of 2 interventions per prescription (range, 0-7) (Table 2). These interventions were made during the initial review or at the time of follow-up. The most common interventions were dose adjustments (19%), clarification of incomplete orders (18%), patient education (18%), and side effect management (17%). Many interventions were rated as significant, and could have greatly impacted patient safety. Table 3 lists the classification criteria for each intervention, along with specific examples for each category.10 Table 4 describes the total number of interventions and the severity associated with each error. Fifty percent of pharmacist interventions were considered to be significant, 31% were very significant, and 10% were extremely significant. An intervention could be considered extremely significant for reasons including preventing a potentially life-threatening event or correcting chemotherapy regimens that were underdosed. Thirty-one percent (n = 26/84) of interventions were considered very significant because they avoided a potentially adverse event by correcting doses for organ impairment (eg, chronic kidney disease, liver dysfunction) or managed a side effect that led to an increase in patient adherence. By reviewing orders and making interventions, pharmacists also corrected 45 medication errors prior to these errors reaching the patient. Of these errors, 7% (n = 3/45) were considered life-threatening; 16% (n = 7/45) were serious.
Time-to-Treatment Initiation
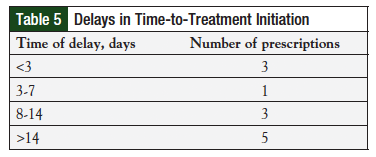
Treatment delays were documented for 16% (n = 12/75) of prescriptions (Table 5). However, start dates were not recorded for prescriptions from the non-PDOC clinics, and the impact of pharmacists on time-to-treatment initiation could not be determined. All 12 prescriptions with documented delays in therapy were from the PDOC clinics. The most common documented reason for delay in treatment initiation was radiation therapy and need for patient financial assistance.
Revenue
Approximately 61% (n = 46/75) of all oral chemotherapy prescriptions were filled at the on-site retail pharmacy, leading to an estimated $106,400 in revenue. The percentage of prescriptions filled at the on-site pharmacy was similar between both groups (P = .55), and each prescription led to approximately $1400 in revenue. Table 6 further describes the revenue generated from each clinic. Because 39% of prescriptions were filled at external pharmacies, approximately $85,000 of potential revenue was missed. Many prescriptions were filled by specialty pharmacies because of insurance requirements.
Discussion
This study of the PDOC is one of the first projects to provide a comprehensive, retrospective review of the role of pharmacists in managing oral chemotherapy. Specifically, we evaluated prescription accuracy using the 2013 ASCO/ONS guidelines, patient safety through pharmacist interventions, procurement through time-to-treatment initiation, and financial benefit through revenue generated at the on-site retail pharmacy. By assessing each of these aspects, this study shows the impact of pharmacists with direct clinic involvement.
Previous research has shown that pharmacists can increase the accuracy of prescribing oral chemotherapy.9,11 An earlier study reviewing capecitabine and temozolomide prescriptions found that pharmacists can make clinically significant interventions by performing double-checks on computerized orders.9 Pharmacists in this setting would receive a daily report of all capecitabine and temozolomide prescriptions ordered within the past 24 hours. They would then review the orders to ensure accurate dosing and compliance with previously studied regimens. If pharmacists found a discrepancy >10%, they notified physicians to correct the orders. Through this review process, pharmacists corrected errors on up to 4% of oral chemotherapy prescriptions. This study provided preliminary evidence to suggest that pharmacists can increase the accuracy of oral chemotherapy orders. However, other reviews along with our study demonstrate that pharmacists can further impact patient care through oral chemotherapy programs. The design of these programs, however, can differ according to institutional practice models.
In an evaluation conducted by Mancini and colleagues, pharmacists at St. Luke's Mountain States Tumor Institute (MSTI) assisted with prescribing oral chemotherapy using a centralized office for 5 oncology clinics.8 In this project, pharmacists reviewed the prescriptions for accuracy, checked laboratory values, evaluated drug interactions, assisted with benefits assessments, and provided patient counseling. In addition, the pharmacists conducted a follow-up phone call to help with side effect management; patients received a weekly call during the first cycle and a monthly phone call thereafter. Although the design of the pharmacist-managed oral chemotherapy program at MSTI is similar to the one presented in this study, there are significant differences between the 2 programs. First, the pharmacist review, benefits assessment, and follow-up phone calls for the PDOC described in this article are provided for all prescriptions regardless of where the prescription was ultimately filled. In addition, the PDOC at our institution is embedded within preexisting clinics, instead of at a separate physical location where providers refer patients. Because of the costs associated with establishing a physical location, the design established in our study may provide an alternative for cancer centers with a preestablished pharmacist presence. These results may also support the hiring of oncology pharmacists for the clinic setting.
This study is also one of the first to evaluate adherence to the 2013 ASCO/ONS guidelines for oral chemotherapy.6 Because the guidelines were created to improve patient safety and accurate prescribing, adherence to these standards serves as an appropriate surrogate marker. However, a potential limitation of this study is that the clinical outcomes associated with the new guidelines have yet to be established. Another limitation is that the 2013 ASCO/ONS guidelines can be open to interpretation. For example, in this study, all prescriptions were required to have a reference to primary literature to meet the reference to dosing standard, even if the medication had a flat dose. This interpretation was based on the institutional standard for traditional chemotherapy. Furthermore, data analysis in this study excluded prescriptions that used flat dosing from the height and weight analysis; including these prescriptions could have changed the statistical significance of this component. Other investigators may not be this stringent when reviewing prescriptions. Similarly, some institutions may not regard agents such as the tyrosine kinase inhibitors as oral chemotherapy; excluding these data could also cause differences in outcomes.
Prescribers in the clinics with the PDOC still did not reach 100% compliance with all of the 2013 ASCO/ONS guidelines. Some of the prescribers did not fill in all of the fields (eg, height, weight, and laboratory values) prior to the prescription leaving the clinic. Although pharmacists reviewed the orders and filled in these values at a later date, these prescriptions were considered to be incomplete for these components. Also, some prescribers may not have notified the pharmacists of a new prescription. Pharmacists within the PDOC now run a daily report to ensure that they have reviewed all oral chemotherapy prescriptions before they are filled.
Pharmacist safety interventions were also reviewed to evaluate the clinical significance of the pharmacist presence. By implementing a tested scale for significance, this study found that pharmacists reconciled clinically significant errors, including ones that were life-threatening. The use of this scale is one of the many strengths of the study, because it further validates the need for pharmacists in managing oral chemotherapy. The pharmacist scoring the interventions was not involved in the data collection, or embedded in the PDOC, which reduced the potential for investigator bias. Even so, the subjectivity of interpreting the scale may cause interrater variability. In addition, the lack of consistency in documentation by the PDOC pharmacists could have decreased the accuracy of the ratings. Some pharmacists provided details regarding the patient case and outcome of intervention, whereas other pharmacists classified the intervention, but did not provide any descriptive information. Despite these constraints, results from this study show that pharmacist review can prevent medication errors and enhance the safe use of oral chemotherapy.
Other limitations of this study include differences in comparison groups and the retrospective design. The PDOC group consisted of patients from the GI and neuro-oncology clinics, whereas the non-PDOC group contained patients with lung, breast, and renal cancers. The differences in pathophysiology and treatment options between these groups could have influenced the prescribing of oral chemotherapy and complexities of the regimens. As the program expands into these clinics, pharmacists will have to determine which prescriptions qualify for review; at this time, the PDOC clinics consider tyrosine kinase inhibitors as chemotherapy and do not include hormonal agents. However, this interpretation may differ among institutions. Another potential limitation of this study is the retrospective design, which relies on the accuracy of the EMR. Specifically, for secondary outcomes, start times were not recorded for non-PDOC prescriptions, and the impact of pharmacists on this outcome could not be determined.
The use of preprinted order sets could also be a limitation; institutions with a complete EMR would have to adjust the PDOC process. At our institution, preprinted order sets were deemed to be safer for initiating the PDOC because EMRs were not designed for chemotherapy; the PDOC is being transitioned to a fully electronic system. Finally, cost and reimbursement of comedications relied on estimation. Although reimbursement data were available for prescriptions filled at the on-site retail pharmacy, controls were used for estimating missed revenue. Reimbursement for prescriptions filled by Medicare Part B was estimated by calculating 80% of the maximum allowable charge–because this is the portion paid by Medicare. This provided a conservative estimate on the impact of revenue generated for the institution by the PDOC. Expanding the inclusion criteria to all oral chemotherapy prescriptions, and not just reviewing the first cycle, could change some of the outcomes associated with revenue.
This study, along with previous reviews,8,11 suggests the need for pharmacists to be involved with evaluating order accuracy within the clinic setting. Although including the 2013 ASCO/ONS requirements on the prescriptions may help external pharmacists verify their accuracy, pharmacists in retail or specialty pharmacy settings often do not have access to the complete medication profile.2 Incomplete medication profiles can prevent pharmacists from accurately identifying drug interactions and appropriate side effect management.
These pharmacists may have difficulty screening for all interactions and reviewing pertinent labs. Therefore, the National Comprehensive Cancer Network Task Force advocates for strong relationships between pharmacists and prescribers to better screen for order accuracy and drug interactions.1
In the current study, PDOC pharmacists reviewed prescriptions for all patients, regardless of where the prescription was ultimately filled. In addition, having an on-site retail pharmacy provides better coordination of care. For example, the on-site retail pharmacy can alert providers if a patient has not refilled a prescription on time, and directly communicate concerns about patient access. Furthermore, for patients who receive all medications at the retail pharmacy, pharmacists can screen for any other drug interactions that might be unknown to the clinic providers.
Although they were not formally quantified or assessed, the physician, nursing, and administrative support for the PDOC clinic was instrumental in expanding this program to provide consistent coverage for numerous other clinics (eg, breast, genitourinary). Finally, this current study shows the profitability of filling oral chemotherapy prescriptions with approximately $106,400 generated for the institution. However, approximately $85,100 was missed because of patients using external pharmacies. By expanding the on-site retail pharmacy to a specialty pharmacy, the organization could capture a portion of the missed revenue.
Conclusion
Through the PDOC, pharmacists have been able to significantly impact patient care by increasing prescription accuracy and safety interventions. Because of the success of this pilot project, the PDOC will be expanded into other oncology-hematology clinics that commonly prescribe oral chemotherapy. Embedding pharmacists in the clinic involved with the prescribing of oral chemotherapy can increase coordination of care and contribute to patient safety.
Author Disclosure Statement
The authors reported no conflicts of interest.
References
1. Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(Suppl 3):S1-S14.
2. McCue DA, Lohr LK, Pick AM. Improving adherence to oral cancer therapy in clinical practice. Pharmacotherapy. 2014;34:481-494.
3. Mathes T, Antoine SL, Pieper D, Eikermann M. Adherence enhancing interventions for oral anticancer agents: a systematic review. Cancer Treat Rev. 2014;40:102- 108.
4. Hede K. Increase in oral cancer drugs raises thorny issues for oncology practices. J Natl Cancer Inst. 2009;101:1534-1536.
5. Charpentier MM, Orr KK, Taveira TH. Improving pharmacist knowledge of oral chemotherapy in the community. Ann Pharmacother. 2012;46:1205-1211.
6. Neuss MN, Polovich M, McNiff K, et al. 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. J Oncol Pract. 2013;9(Suppl 2):5s-13s.
7. Collins CM, Elsaid KA. Using an enhanced oral chemotherapy computerized provider order entry system to reduce prescribing errors and improve safety. Int J Qual Health Care. 2011;23:36-43.
8. Mancini R, Kaster LM, Vu B, et al. Implementation of a pharmacist-managed interdisciplinary oral chemotherapy program in a community cancer center. J Hematol Oncol Pharm. 2011;1:23-30.
9. Jatoi A, Smith EL, Gunderson HD, et al. Capecitabine and temozolomide: design, implementation, and preliminary outcomes from a pilot project to ensure safe prescribing of oral chemotherapy. J Oncol Pract. 2010;6:210-212.
10. Overhage JM, Lukes A. Practical, reliable, comprehensive method for characterizing pharmacists’ clinical activities. Am J Health Syst Pharm. 1999;56:2444-2450.
11. Mancini R, Wilson D. A pharmacist-managed oral chemotherapy program: an economic and clinical opportunity. Oncol Issues. 2012;28-31.