Targeting the human epidermal growth factor receptor (HER)2 protein has proved efficacy for the treatment of breast, gastric, and gastroesophageal cancers. Trastuzumab, a monoclonal antibody directed against HER2, has been approved by the US Food and Drug Administration for the treatment of patients with these cancer types.1 The rate of severe hypersensitivity reactions with trastuzumab, such as respiratory distress, hypotension, and swelling, is relatively rare (0.6%), although these events can be fatal.1
Pertuzumab, another HER2-targeting monoclonal antibody used in the treatment of breast cancer, is associated with an infusion reaction rate ranging from 13% to 21% (all grades), with <1% constituting grade 3 or 4 reactions.2
Ado-trastuzumab emtansine, a monoclonal antibody conjugate of trastuzumab and the microtubule inhibitor DM1 (DM1 plus the maleimidomethyl cyclohexane-1-carboxylate linker are referred to as emtansine), has a hypersensitivity reaction rate of 1.4%.3
The details of the mechanism(s) of hypersensitivity reactions to monoclonal antibody HER2-targeting therapies are still unclear, but may not be mediated by nonimmunoglobulin (Ig)E. The prescribing information for trastuzumab, for pertuzumab, and for ado-trastuzumab emtansine do not recommend routine premedication for the prevention of hypersensitivity, but they do provide several strategies for managing infusion reactions.1-3 These strategies include decreasing the rate of infusion or adding premedication; however, for all 3 drugs, permanent discontinuation of treatment is recommended for life-threatening infusion-related reactions.1-3 Halting or changing treatment, however, is not always ideal if there is no acceptable therapy alternative.
Desensitization protocols for various monoclonal antibodies are represented in the literature, but few publications address trastuzumab and ado-trastuzumab emtansine desensitization procedures.4-11
We report the successful use of desensitization protocols for outpatient treatment with trastuzumab and subsequent treatment with ado-trastuzumab emtansine in a 52-year-old woman with metastatic breast cancer.
Case Report
Our patient was aged 39 years when she was initially diagnosed with stage IIA hormone receptor (HR)-positive, HER2-positive invasive ductal breast cancer. She had surgery and received adjuvant chemotherapy that included trastuzumab for 12 weeks, radiation therapy, and 5 years of endocrine therapy. Approximately 10 years later, the woman was diagnosed with biopsy-confirmed HR-negative, HER2-positive metastatic adenocarcinoma.
The patient received the first cycle of pertuzumab, trastuzumab, and docetaxel at an outside facility in an outpatient infusion center, as well as premedication with acetaminophen, diphenhydramine, and dexamethasone. She completed the pertuzumab infusion without any issues, but she had mild chest tightness during the trastuzumab infusion, which was not pronounced enough to report to the infusion nurse. The administration of docetaxel was uneventful. When she left the infusion area, the patient noted a mild rash, stomatitis, and joint pain, which persisted for 36 hours.
Three weeks later, she received the same 3 premedications before cycle 2; however, within 5 minutes of the pertuzumab infusion, the patient had facial flushing, shortness of breath, tachycardia, and chest pain. She was given oxygen and intravenous diphenhydramine, hydrocortisone, and famotidine, with some immediate relief of symptoms, but she was transported to the emergency department for the management of continued chest pain and hypotension. The patient was subsequently hospitalized for overnight observation as a result of lingering chest discomfort and was discharged the next morning. The second doses of trastuzumab and docetaxel were not given.
The patient then transferred her care to our institution. We made a clinical decision to alter the taxane used in her treatment from docetaxel to paclitaxel. We also prepared a plan for the patient to receive a trial treatment of trastuzumab, followed by paclitaxel on the next day. The premedication used before trastuzumab treatment included diphenhydramine and ranitidine, and the infusion time was modified from a standard 30-minute infusion to a slow titration over approximately 4 hours.
During the last 10 mL of the first dose of trastuzumab, the patient had shortness of breath, chest tightness, chest pressure, and pruritus. The infusion was stopped, and she received intravenous diphenhydramine and hydrocortisone, with complete resolution of her symptoms. Six days later, the patient received premedication with diphenhydramine, ranitidine, and dexamethasone, and she then tolerated single-agent paclitaxel without issues.
The significant impact that HER2-targeted therapy can have on clinical outcomes prompted consultation with an allergist. Although the patient had previously tolerated treatment with trastuzumab a decade earlier, as well as her initial dose of trastuzumab at an outside institution, the flushing and dyspnea she had during the recent trastuzumab infusion and her life-threatening reaction to pertuzumab warranted extreme caution.
Skin testing for trastuzumab and pertuzumab were not available at the time of the consultation and would not have altered the recommendation to proceed with desensitization in this case, because of the patient’s history of severe allergic reaction.
Desensitization to trastuzumab using a 3-bag, 12-step protocol was recommended based on a general approach to monoclonal antibody desensitization in the literature, and the previously reported desensitizations to trastuzumab.4-11 Approximately 10 minutes into step 12 of the desensitization protocol (after 75 mg of trastuzumab was administered), the patient reported shortness of breath and chest pain with tachycardia. The infusion of trastuzumab was stopped, and she received intravenous diphenhydramine and hydrocortisone. Thirty minutes after her initial symptoms started, the patient’s chest pain returned and prompted the administration of intramuscular epinephrine. Her symptoms improved within minutes and completely resolved in 15 minutes. The remainder of the trastuzumab dose was not given.
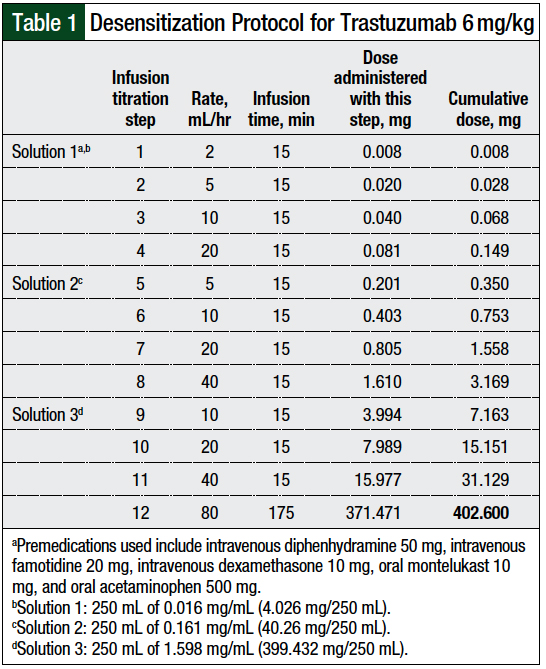
Because of the significant reaction the patient had with the first attempt at a 12-step desensitization, and her wish to transition to every-3-week dosing of trastuzumab, the trastuzumab desensitization protocol and the premedications were modified (Table 1).
In contrast to the initial desensitization attempt, the premedication regimen included intravenous diphenhydramine and famotidine, rather than oral drugs, and montelukast and acetaminophen were added to the treatment regimen. These alterations were decided based on collaboration among the pharmacy, allergy, and medical oncology clinicians.
Three infusion solutions—solutions 1, 2, and 3—contained 1%, 10%, and 99%, respectively, of the total trastuzumab dose. Solution 1 was used for infusion rate titration step 1 to step 4, solution 2 was used for infusion rate titration step 5 to step 8, and solution 3 was used for infusion rate titration step 9 to step 12.
The rate of the infusion was adjusted every 15 minutes, and each step delivered approximately twice the dose of trastuzumab than the previous step. The final step, step 12, was continued until the total dose was infused. The patient tolerated treatment with trastuzumab using this desensitization regimen and was able to receive 10 additional doses of trastuzumab using this protocol.
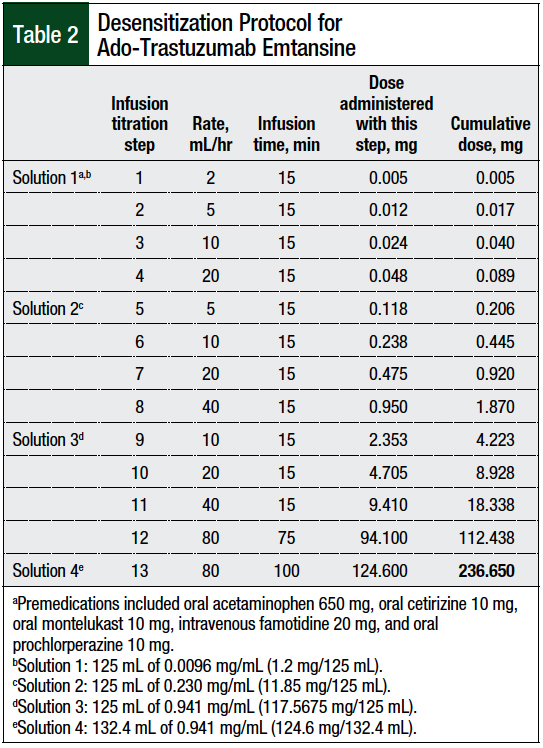
Progressive tumor burden prompted a change to ado-trastuzumab emtansine therapy. The prescribing information of ado-trastuzumab emtansine recommends against its use if the patient has had previous hypersensitivity to treatment with trastuzumab.3 Because of the patient’s history of anaphylactic reactions to treatment with pertuzumab and trastuzumab, the decision was made to follow a desensitization protocol (Table 2).
The 3-bag, 12-step approach detailed in published articles on monoclonal antibody desensitizations4-6,8-11 was modified to a 4-bag, 13-step protocol, because of the short stability at room temperature of ado-trastuzumab emtansine and the paucity of data specific to ado-trastuzumab emtansine desensitization.3,10
Four infusion solutions—solutions 1, 2, 3, and 4—contained 0.5%, 5%, 50%, and 52%, respectively, of the total dose. Solution 1 was used for infusion rate titration step 1 to step 4, solution 2 for infusion rate titration step 5 to step 8, solution 3 for infusion rate titration step 9 to step 12, and solution 4 for infusion rate titration step 13.
The rate of the infusion was adjusted every 15 minutes, and each step delivered approximately twice the dose of the previous step, with the exception of step 13, which continued the same rate as in step 12, but with a new bag. The final step, step 13, was continued until the total dose was infused.
Our patient had no symptoms during the first ado-trastuzumab emtansine infusion (cycle 1), but 3 to 7 hours after the infusion, she had shortness of breath, tachypnea, and tachycardia, and required oxygen and corticosteroids, which resolved all her symptoms. Consequently, treatment cycles 2 and 3 were administered in the hospital and were well-tolerated.
However, imaging after the third cycle of ado-trastuzumab emtansine treatment demonstrated disease progression, and ado-trastuzumab emtansine therapy was discontinued. Subsequent therapies were poorly tolerated by the patient, and she was transitioned to hospice care.
Discussion
Several publications describe the 3-bag, 12-step trastuzumab desensitization protocols used for our patient.4,8,9,11 Brennan and colleagues reviewed 3 patients with breast cancer who had mild-to-moderate hypersensitivity reactions to treatment with trastuzumab.4 Similar to our patient, these reactions occurred with re-exposure to trastuzumab after a prolonged, well-tolerated course of therapy. Unlike our patient, skin testing (epicutaneous or intradermal) was completed and was positive for all 3 patients.4 All 3 patients successfully underwent 8 to 11 courses of additional therapy using desensitization infusions. Breakthrough symptoms occurred during 20% of the total 29 desensitizations completed.4
Lee and colleagues reported the case of a patient with metastatic breast cancer who received trastuzumab desensitization.8 The patient was able to receive 10 palliative treatment cycles using a desensitization protocol with hypersensitivity symptoms on only 2 of those 10 courses. A skin test was not performed.8
Bavbek and colleagues described a patient with metastatic breast cancer who had a grade 3 hypersensitivity reaction during her first infusion of trastuzumab.9 This patient had a negative skin test result and completed 1 desensitization treatment in the inpatient setting without noted complications.9 In addition, de Lira-Quezada and colleagues detailed a patient with stage IV breast cancer who had an initial anaphylactic reaction to treatment with trastuzumab and a subsequent positive skin test.11 The patient underwent 20 rapid desensitization treatments without noted reactions,11 after receiving the same desensitization approach used for our patient.
Overall, the reported use of skin tests to direct desensitization to trastuzumab have been variable.4,8,9,11 This might have resulted from a lack of standardized use of reliable and accurate skin testing, the need for a skin test to be performed at least 3 weeks after the initial allergic reaction, to reduce false-negative test results, or the general recommendation that if an allergic reaction is severe enough, a desensitization regimen would be recommended if there is a need to continue the reaction-inducing agent, regardless of the results of a skin test.4
Our patient’s severe reaction to treatment with pertuzumab and trastuzumab necessitated desensitization to allow for continued treatment.
Finally, a hypersensitivity reaction to several HER2 monoclonal antibody therapies in a single patient raises the question of cross-reactivity between these agents. Our patient reacted to treatment with pertuzumab, trastuzumab, and ado-trastuzumab emtansine.
The mechanism of possible cross-reactivity is unknown, but it could be linked to the murine humanization of these 3 molecules. All 3 monoclonal antibodies are produced in a mammalian cell (Chinese hamster ovary) culture, using recombinant DNA technology.1-3 As such, there is the possibility of common murine epitopes.4 Further reports are needed to determine whether cross-reactivity exists, and if that is the case, to ascertain its mechanism.
Conclusion
The reactions to HER2 monoclonal antibody therapies in our patient raise the question of cross-reactivity between these agents. Skin testing could be a valuable tool to evaluate cross-sensitivity in this setting, because IgE sensitization would be indicated by a positive skin test result, although skin testing is not uniformly used.
Overall, this case report supports the use of a multidisciplinary approach involving medical oncology, allergy, pharmacy, and infusion center staff to implement drug desensitization for medications when an equally effective treatment alternative is not available.
Author Disclosure Statement
Dr Kempke, Dr Kraft, Dr Castells, and Dr Ravikumar have no conflicts of interest to report. Dr Van Poznak received research support to her institution from Bayer.
References
- Herceptin (trastuzumab) for injection, for intravenous use [prescribing information]. San Francisco, CA: Genentech; November 2018.
- Perjeta (pertuzumab) injection, for intravenous use [prescribing information]. San Francisco, CA: Genentech; January 2020.
- Kadcyla (ado-trastuzumab emtansine) for injection, for intravenous use [prescribing information]. San Francisco, CA: Genentech; September 2020.
- Brennan PJ, Rodriguez Bouza T, Hsu FI, et al. Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol. 2009;124:1259-1266.
- Galvão VR, Castells MC. Hypersensitivity to biological agents—updated diagnosis, management, and treatment. J Allergy Clin Immunol Pract. 2015;3:175-185; quiz 186.
- Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122:574-580.
- Melamed J, Stahlman JE. Rapid desensitization and rush immunotherapy to trastuzumab (Herceptin). J Allergy Clin Immunol. 2002;110:813-814.
- Lee CW, Matulonis UA, Castells MC. Rapid inpatient/outpatient desensitization for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol. 2005;99:393-399.
- Bavbek S, Kendirlinan R, Çerçi P, et al. Rapid drug desensitization with biologics: a single-center experience with four biologics. Int Arch Allergy Immunol. 2016;171:227-233.
- Hong DI, Bankova L, Cahill KN, et al. Allergy to monoclonal antibodies: cutting-edge desensitization methods for cutting-edge therapies. Expert Rev Clin Immunol. 2012;8:43-52; quiz 53-54.
- de Lira-Quezada CE, Villarreal-González RV, González-Díaz SN, Acuña-Ortega N. Protocol for desensitization to trastuzumab in a patient with anaphylaxis and stage IV breast cancer: a case report. J Investig Allergol Clin Immunol. 2020;30:376-377.