Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in patients with or without active cancer.1,2 The incidence of VTE ranges from 300,000 to 600,000 events annually, with cumulative recurrence rates of 25% at 5 years and 36% at 10 years from the index event in the absence of long-term anticoagulation.1,3
The incidence of VTE in patients with active cancer approaches 6 per 100 person-years in observational cohort studies, with a recurrence rate of 9.6 per 100 person-years.2 The increased use of long-term anticoagulation with a vitamin K antagonist is thought to underpin the decline in recurrent VTE rates from 17% to 9% between 1999 and 2009.3
Obesity is an emerging worldwide problem. It is estimated that by 2030, nearly 50% of adults will be classified as obese, defined as a body mass index (BMI) of ≥30 kg/m2, and approximately 24% of adults will be classified as severely obese (BMI ≥35 kg/m2).4 Obesity is associated with an increased risk for atrial fibrillation and VTE, both of which often necessitate anticoagulation therapy.5
Direct-acting oral anticoagulants (DOACs) represent a convenient alternative to warfarin in the treatment of VTE, given their fixed-dosing regimens and limited dietary interactions, without the need for routine clinical laboratory monitoring. DOACs have become the first-line anticoagulant for VTE in the general population; however, their fixed-dosing strategy has raised safety and efficacy concerns regarding the use of DOACs in the obese population.6 Additional research in this area is warranted.
Pharmacokinetic studies of DOACs in the obese population have shown conflicting results.7,8 An open-label, single-dose study comparing the pharmacokinetics of apixaban in 54 healthy volunteers demonstrated 31% lower mean peak apixaban plasma concentrations and a 23% lower area under the curve (AUC) in patients who weighed ≥120 kg compared with patients who weighed 65 kg to 85 kg.7 By contrast, another study that investigated the pharmacokinetics of single-dose rivaroxaban in 48 healthy volunteers showed that peak plasma concentrations and the AUC were unaffected in patients weighing >120 kg compared with those weighing 70 kg to 80 kg.8
Outside of pharmacokinetic studies, clinical data on the use of DOACs in obese patients are limited. To date, no randomized controlled trials or large prospective studies have investigated the safety and efficacy of DOACs in obese populations. Only half of the phase 3 clinical trials comparing the safety and efficacy of DOACs and warfarin in the treatment of VTE included a subgroup analysis by weight.9-11 However, these subgroup analyses are limited by inconsistencies in weight cutoffs, BMI stratifications, and inclusion of relatively low percentages (14%-19%) of obese patients.9-14
The majority of these studies defined obesity as a lower cutoff weight of 90 kg to 100 kg. Although DOACs were found to be noninferior to warfarin in the outcome of recurrent VTE, which was similar to reduced bleeding events in the general population,9-14 it is unclear whether these results may be applied to the obese population.
Given concerns for the efficacy and safety of DOACs in obese patients or in patients with high body weight, the current guidelines from the International Society on Thrombosis and Haemostasis recommend against the use of DOACs in patients with a BMI >40 kg/m2 or a body weight exceeding 120 kg.15 However, in clinical practice, obese or high–body weight patients often receive DOACs for a variety of reasons (eg, patient preference, unstable international normalized ratio [INR]).
We conducted a retrospective study to investigate the efficacy and safety of DOACs for the treatment of acute VTE in obese patients with and without active cancer. The purpose of our study was to compare the rates of recurrent VTE and major bleeding in patients with high body weight (≥120 kg) and in obese patients (BMI ≥40 kg/m2) who received DOACs with patients who started treatment with unfractionated heparin or low-molecular-weight heparin (LMWH), followed by warfarin, for acute VTE.
Methods
The study included patients aged 18 to 89 years with a BMI of ≥40 kg/m2 or body weight of ≥120 kg, who underwent treatment for objectively confirmed, symptomatic or asymptomatic VTE with a DOAC (ie, apixaban or rivaroxaban) or warfarin at The Ohio State University Wexner Medical Center and The Arthur G. James Cancer Hospital and Richard J. Solove Research Institute in Columbus, OH, between January 1, 2010, and July 31, 2017. This study was approved by the university’s Institutional Review Board.
Patients were excluded from the study if they had any of the following conditions: thrombectomy or systemic- or catheter-directed thrombolytic therapy for the index VTE; requirement for current long-term dual antiplatelet therapy with aspirin ≥81 mg daily and a direct-acting P2Y12 inhibitor (ie, clopidogrel, prasugrel, or ticagrelor); indication other than VTE for anticoagulation therapy; creatinine clearance of <30 mL/min, as calculated by the Cockcroft-Gault formula, or a serum creatinine of ≥2.0 mg/dL; initiated anticoagulation >1 week after the diagnosis of the index VTE; absence of ≥2 documented follow-up encounters within the electronic medical record within a 24-month period; a platelet count of <50 × 109/L; documented thrombophilia; a known contraindication to heparin; or were prisoners or pregnant.
A proximal deep-vein thrombosis (DVT) was confirmed by the presence of a noncompressible proximal vein (involving the popliteal vein or more proximal) on compression ultrasonography or by an intraluminal filling defect of a proximal vein on computed tomography or magnetic resonance venography.
The presence of a pulmonary embolism was confirmed by a perfusion defect of ≥75% of a segment with a local normal ventilation result on a ventilation-perfusion lung scan, an intraluminal filling defect in segmental or more proximal branches on spiral computed tomography, or an intraluminal filling defect or sudden occlusion of vessels ≥2.5 mm in diameter on pulmonary angiography.
High body weight was defined as an actual body weight of >120 kg. Because patients with an actual body weight of >120 kg may have had a BMI of <40 kg/m2, obesity (defined as BMI >30 kg/m2) was further divided into 3 grades, based on the BMI grade (grade 1, 30-<35 kg/m2; grade 2, 35-<40 kg/m2; and grade 3, ≥40 kg/m2).
Data were collected for 24 months from the date of the index VTE and included the patients’ baseline demographics and the clinical characteristics of the index VTE, recurrent VTE events, major bleeding event, and clinically relevant nonmajor bleeding events. The patients’ demographics included age, race, sex, body weight, and BMI. The clinical characteristics of the index VTE event included the baseline hemoglobin level, platelet count, serum creatinine, creatinine clearance, and INR at the time of the index VTE; the documented VTE diagnosis (DVT, pulmonary embolism, DVT and pulmonary embolism, other thrombotic event); the location of the VTE (proximal or distal); the presence of a vena cava filter; the etiology of the VTE (unprovoked or provoked by risk factors, such as recent surgery or trauma, immobilization of >3 days, line-associated, estrogen-associated, cancer-related, or critical care–associated); and a history of VTE.
Outcome Measures
The primary outcome was the composite of recurrent VTE and major bleeding. Recurrent VTE was defined as an objectively confirmed new thrombosis of a site that was either previously uninvolved or had interval documentation of VTE resolution after systemic anticoagulation. Major bleeding event was defined by the standard International Society on Thrombosis and Haemostasis criteria, which is overt bleeding associated with a decrease in hemoglobin level by ≥2 g/dL, bleeding requiring the transfusion of ≥2 units of packed red blood cells, bleeding occurring in a critical site (eg, subdural, epidural, subarachnoid, intracerebral, hemorrhagic transformation of an ischemic stroke), or bleeding contributing to death.16
The secondary outcomes included each individual component of the primary outcome and a composite of major bleeding and a clinically relevant nonmajor bleeding event, which was defined as overt bleeding that does not meet the criteria for major bleeding but requiring medical intervention, an unscheduled physician office visit, interruption of anticoagulation, or discomfort or impairment in carrying out activities of daily living.17
Statistical Methods
Assuming a 9% incidence rate of recurrent VTE and major bleeding in 24 months, a sample size of 200 patients within each treatment arm would be required to confer 80% power to detect a 20% difference in the primary composite outcome.3 Continuous parametric data were presented as means (± standard deviations) and were analyzed using the Student’s t-test.
Continuous nonparametric data were presented as medians (25%-75% interquartile range) and were analyzed using the Mann–Whitney U test. Nominal data were presented as frequency and percentages, and were analyzed using the Fisher’s exact test or chi-square test, as appropriate. Two-tailed statistical tests were used, with a significance level set at P <.05. All statistical analyses were conducted using SPSS for Windows version 26 (IBM; Armonk, NY).
Results
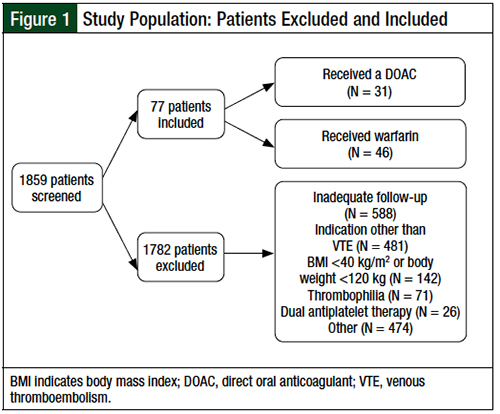
A total of 1859 patients who received treatment with a DOAC or warfarin between January 1, 2010, and July 31, 2017, were screened for inclusion (Figure 1). Of these 1859 patients, 77 obese patients or patients with high body weight who received a DOAC (N = 31) or warfarin (N = 46) for an objectively confirmed VTE were eligible for inclusion.
The primary reasons for exclusion included inadequate follow-up, as defined by the absence of at least 2 documented encounters within 24 months of their index VTE event (32%), an indication for anticoagulation other than VTE (26%), a BMI of <40 kg/m2 or an actual body weight of <120 kg (8%), documented thrombophilia (4%), dual antiplatelet therapy (1.4%), and initiation of anticoagulation >1 week after a diagnosis of index VTE (1.3%).
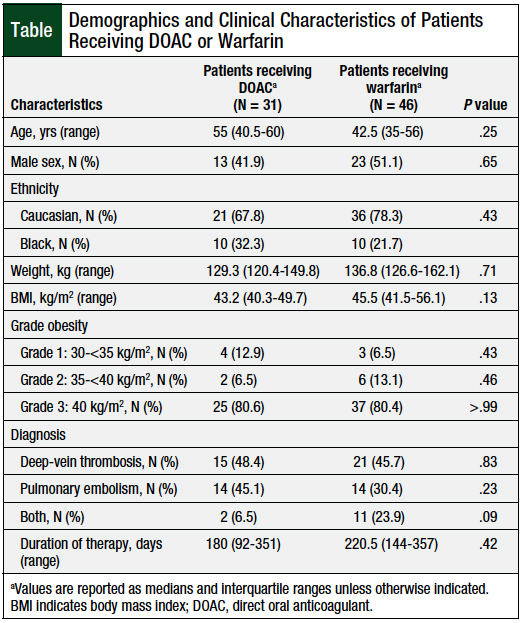
The baseline demographics were similar between the 2 groups (Table). The patients’ median age was 46 years (35-59.5 years), the median body weight was 131.5 kg (124-156.3 kg), with a median BMI of 44.4 kg/m2 (41.5-52 kg/m2).
The baseline mean serum creatinine was similar between the DOAC and the warfarin groups (0.89 + 0.29 mg/dL vs 0.93 + 0.31 mg/dL, respectively; P = .54). The index VTE was provoked in most cases (77.9%) with DVT diagnosed in 46.7% of the patients, pulmonary embolism in 36.4%, and concomitant DVT and pulmonary embolism in 16.9%.
Approximately 20% of the patients had a history of VTE, which was not significantly different between the DOAC and the warfarin groups (12.9% vs 23.9%, respectively; P = .26). In all, 13% of the total patients had an active malignancy, which was not significantly different between the DOAC and the warfarin groups (19.4% vs 8.7%, respectively; P = .19) A total of 3 (6.5%) patients in the warfarin group had a vena cava filter at the time of their index VTE versus none in the DOAC group (P = .27).
The median duration of anticoagulation therapy was approximately 6 months, with no significant difference between the DOAC and the warfarin groups: 180 days (range, 92-351 days) versus 220.5 days (range, 144-357 days; P = .42). For patients receiving a DOAC, apixaban and rivaroxaban were used in 29% and 71% of the patients, respectively. DOACs were prescribed at standard doses per each drug’s prescribing information: apixaban 10 mg twice daily for 7 days, followed by 5 mg twice daily; rivaroxaban 15 mg twice daily for 21 days, followed by 20 mg daily.
Drug-specific anti–factor Xa levels were not routinely monitored at our institution. The median total weekly dose of warfarin at initiation was 35 mg (range, 35-61.3 mg). The median number of documented visits for INR monitoring was 11 (range, 4.5-20), and the median percentage of time in therapeutic range was 47.1% (range, 30.3%-57%).
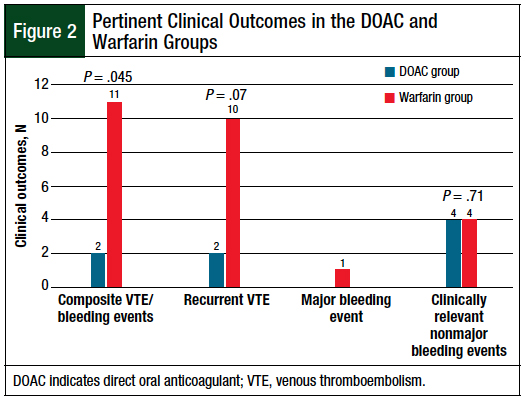
The patients who were receiving DOACs had significantly reduced rates of the primary composite outcome of recurrent VTE or a major bleeding event compared with the patients who received warfarin (6.5% vs 23.9%, respectively; P = .045; Figure 2). There was a trend toward a reduced rate of recurrent VTE in the patients who received a DOAC compared with those who received warfarin (6.5% vs 21.7%, respectively; P = .07; Figure 2).
In the DOAC group, recurrent VTE occurred in 11.1% of patients receiving apixaban and in 4.8% of those receiving rivaroxaban (P = .5). A major bleeding event occurred in 1 patient who received warfarin, which was an intracerebral hemorrhage. The rates of clinically relevant nonmajor bleeding were low and were similar between the DOAC and the warfarin groups (12.5% vs 8.7%, respectively; P = .71; Figure 2).
Of the patients who received a DOAC, 2 had an unscheduled office visit for hematuria, 1 had an unscheduled office visit for menstrual bleeding, and 1 went to the emergency department for hematuria. For those who received a DOAC, clinically relevant nonmajor bleeding occurred in none of the patients who received apixaban and in 18.2% of the patients who received rivaroxaban (P = .3).
For patients who received warfarin, 2 patients went to the emergency department, 1 for hematuria and 1 for bleeding from wounds.
Discussion
In the cohort of obese patients and patients with high body weight in our study, a significantly lower rate of the composite outcome of recurrent VTE and major bleeding was reported in patients who received a DOAC for acute VTE compared with those who received warfarin. This difference was largely driven by a reduction in the rate of recurrent VTE. Only 1 patient had a major bleeding event, and the rates of clinically relevant nonmajor bleeding events were similar between the 2 groups.
Although our study did not include a homogenous population of patients with active cancer, the results of our analysis may assist in guiding the clinical application of apixaban and rivaroxaban in obese patients or in patients with high body weight who have active cancer, in accordance with recent randomized controlled trials that compared DOACs with LMWHs for the treatment of VTE associated with cancer.18,19
In a multicenter, randomized, open-label pilot study in the United Kingdom, treatment with rivaroxaban for a total of 6 months was associated with a significant reduction in the primary outcome of recurrent VTE versus treatment with dalteparin.18 In this study of 203 patients with active cancer, rivaroxaban dosed per the prescribing information was compared with dalteparin 200 IU/kg daily for 1 month, followed by 150 IU/kg daily for months 2 to 6 (4% vs 11%, respectively; hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19-0.99). The corresponding rates of major bleeding events (6% vs 4%, respectively; HR, 1.83; 95% CI, 0.68-4.96) and clinically relevant nonmajor bleeding events (13% vs 4%, respectively; HR, 3.76; 95% CI, 1.63-8.69) were higher in patients who received rivaroxaban than in patients who received dalteparin.18
In a multinational, randomized, open-label, noninferiority trial of 1155 patients with active cancer and newly diagnosed symptomatic or incidental VTE, treatment with apixaban was noninferior to dalteparin in the primary outcome of recurrent VTE.19 The rates of major bleeding events were similar between the apixaban and the dalteparin treatment groups (3.8% vs 4%, respectively; P = .6).19
It is unclear how these results may be applied to obese patients or patients with high body weight who have active cancer, because the median BMI and mean weight of the enrolled patients in each of these studies was 26.6 kg/m2 and 75 kg, respectively.18,19
The impact of morbid obesity on warfarin dosing has been well-described in the literature.20,21 Mueller and colleagues showed a positive correlation between BMI and the total weekly dose of warfarin in a retrospective analysis of 831 patients with a BMI of 13.4 kg/m2 to 63.1 kg/m2.20 They reported that for every 1-point increase in BMI, the total weekly dose of warfarin increased by 0.69 mg.20 Tellor and colleagues also reported a higher total weekly dose of warfarin in morbidly obese (41.5 mg) patients compared with normal or overweight patients (28.8 mg; P <.05) and obese (32.4 mg; P <.05) patients.21
Many studies that compare DOACs with warfarin across BMI categories have been conducted in patients with atrial fibrillation. In a study by Zhou and colleagues, the investigators concluded that overweight patients (BMI 25-<30 kg/m2) receiving a DOAC had significantly lower rates of stroke or systemic embolism and major bleeding compared with patients who received warfarin in a meta-analysis of 9 studies of patients with atrial fibrillation.22 Obese patients (BMI ≥30 kg/m2) had no higher risk for stroke or systemic embolism and major bleeding.22
Kushnir and colleagues conducted a single-center retrospective study of patients with a BMI of ≥40 kg/m2 (median, 44.5 kg/m2) who received apixaban (N = 150), rivaroxaban (N = 326), or warfarin (N = 319) for the treatment of atrial fibrillation (N = 429) or VTE (N = 366).23 For patients who had atrial fibrillation, the incidence of stroke was 1% with apixaban, 2.3% with rivaroxaban, and 1.3% with warfarin. Major bleeding occurred in 2.9% of patients receiving apixaban or rivaroxaban, and in 7.9% of patients receiving warfarin. For patients who received treatment for VTE, the incidence of recurrent VTE was similar between the apixaban, rivaroxaban, and warfarin groups (2.1%, 2%, and 1.2%, respectively; P = .74), with comparable rates of major bleeding (2.1%, 1.3%, and 2.4%, respectively; P = .77).23
The difference in the incidence of recurrent VTE noted between our analysis and that of Kushnir and colleagues is difficult to explain, because VTE risk factors and time in therapeutic range for the patients who received warfarin were not reported in their study. As noted, the suboptimal time in therapeutic range in our study could partially contribute to these differences. For patients receiving a DOAC, the rates of recurrent VTE in our study were similar to the rates reported in phase 3 pivotal trials with apixaban and rivaroxaban (approximately 2%-3% annually).9,12,13
Coons and colleagues conducted a retrospective study of 1840 patients with a body weight of >100 kg who received a DOAC (rivaroxaban, N = 580; apixaban, N = 33; dabigatran, N = 19) or warfarin (N = 1208) for the treatment of VTE.24 The median BMI was approximately 39 kg/m2, with 45% of the patients having a BMI of >40 kg/m2. Similar to our analysis, approximately 20% of the patients in their study had a history of VTE. In the patients who received a DOAC or warfarin, the rates of recurrent VTE (6.5% vs 6.4%, respectively; P = .93) and bleeding (1.7% vs 1.2%, respectively; P = .31) were similar at 12 months.24
Because VTE and bleeding events were identified by International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification codes as the admitting diagnosis, these rates might have been over- or underestimated in the analysis by Coons and colleagues, given the risk for inaccurate coding.24 Finally, it is possible that the lower proportion of patients with a BMI of <40 kg/m2 (55%) included in this analysis might have led to an increased time in therapeutic range and to lower rates of recurrent VTE compared with our analysis; however, information pertinent to the management of warfarin, including the total weekly dose or time in therapeutic range, was not reported in their analysis.24
In our study, no difference was seen in major bleeding or clinically relevant nonmajor bleeding events between the patients who received a DOAC and those who received warfarin. Similar to other studies comparing DOACs and warfarin,9,12,13,22,23 the patients in our study had a low rate of major bleeding.
Kushnir and colleagues reported a rate of clinically relevant nonmajor bleeding events of 2.2% for apixaban, 7.9% for rivaroxaban, and 7.8% for warfarin in patients with VTE.23 These results are similar to the rates in our study of 0% for apixaban, 18.2% for rivaroxaban, and 8.7% for warfarin. The lower rates of clinically relevant nonmajor bleeding events observed in the patients who received apixaban may be explained by small pharmacokinetic studies in which the peak concentration of apixaban, but not of rivaroxaban, was 31% lower in patients weighing ≥120 kg7,8; however, more definitive studies in obese patients are needed.
Limitations
Our study has several limitations. We calculated that a sample size of 200 patients within each treatment group would be desired. Although we screened more than 1800 patients, only 77 patients met our prespecified inclusion criteria; therefore, our study was underpowered to detect a statistically significant difference in our primary composite outcome. Given the retrospective nature of this study, we were limited to the available records.
Despite our small sample size, a significantly reduced rate of the composite outcome of recurrent VTE and major bleeding events was observed in the DOAC group compared with the warfarin group. In addition, the reliability of the data collection might have been affected by variability in documentation within the electronic medical records.
Our analysis was potentially subject to prescribing bias because the choice of anticoagulant was at the discretion of each clinician, although there were no significant differences in the patients’ characteristics. The primary DOAC prescribed in this population was rivaroxaban (71%), which was likely influenced by the available pharmacokinetic studies.7,8
In addition, The Ohio State University Wexner Medical Center and The Arthur G. James Cancer Hospital and Richard J. Solove Research Institute do not have a specified protocol for dosing warfarin in obese patients, which might have contributed to the suboptimal time in therapeutic range and the higher rate of VTE recurrence observed in the warfarin group.
In our study, patients taking warfarin received a low total weekly dose (median, 35 mg), despite a predominantly (80%) morbidly obese patient population. Although we observed a time in therapeutic range of only 47.1%, with a median of 11 visits for INR monitoring over 24 months, our study did not capture INR monitoring conducted at external institutions, and therefore, these values were likely underestimated. Follow-up INR monitoring and warfarin dosing changes outside our health system were not captured.
Finally, the study’s single-center design may limit the generalizability of our findings to other institutions.
Conclusion
Our findings showed that obese patients or patients with high body weight who received a DOAC had a reduced rate of a composite outcome of recurrent VTE and major bleeding events compared with patients who received warfarin. The less-than-optimal management of warfarin could have contributed to the increased rate of recurrent VTE in the warfarin group, but this reflects a real-world practice and presents challenges in the management of warfarin. Clinically relevant nonmajor bleeding events were comparable between the treatment groups.
These results may assist in guiding the clinical application of apixaban and rivaroxaban in obese patients or in patients with high body weight who have active cancer. Large prospective cohort studies or randomized controlled trials are needed to further characterize the safety and efficacy of DOACs in obese or overweight patients.
Author Disclosure Statement
Dr Cook, Dr Wiczer, Dr Gerlach, Dr Linger, Ms Ritchey, Dr Zickefoose, Dr Wang, and Dr Brower have no conflicts of interest to report.
References
- Khan F, Rahman A, Carrier M, et al; for the MARVELOUS collaborators. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. BMJ. 2019;366:l4363.
- Cohen AT, Katholing A, Rietbrock S, et al. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer: a population-based cohort study. Thromb Haemost. 2017;117:57-65.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- Pomp ER, le Cessie S, Rosendaal FR, Doggen CJM. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139:289-296.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest. 2016;149:315-352.
- Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76:908-916.
- Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47:218-226. Erratum in: J Clin Pharmacol. 2008;48:1366-1367.
- Agnelli G, Buller HR, Cohen A, et al; for the AMPLIFY investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
- Di Nisio M, Vedovati MC, Riera-Mestre A, et al. Treatment of venous thromboembolism with rivaroxaban in relation to body weight: a sub-analysis of the EINSTEIN DVT/PE studies. Thromb Haemost. 2016;116:739-746.
- Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406-1415. Erratum in: N Engl J Med. 2014;370:390.
- EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
- EINSTEIN-PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
- Schulman S, Kearon C, Kakkar AK, et al; for the RE-COVER study group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
- Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313.
- Schulman S, Kearon C; for the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-694.
- Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; for the Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119-2126.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36:2017-2023.
- Agnelli G, Becattini C, Meyer G, et al; for the Caravaggio investigators. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382:1599-1607.
- Mueller JA, Patel T, Halawa A, et al. Warfarin dosing and body mass index. Ann Pharmcother. 2014;48:584-588.
- Tellor KB, Nguyen SN, Bultas AC, et al. Evaluation of the impact of body mass index on warfarin requirements in hospitalized patients. Ther Adv Cardiovasc Dis. 2018;12:207-216.
- Zhou Y, Ma J, Zhu W. Efficacy and safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation across BMI categories: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2020;20:51-60.
- Kushnir M, Choi Y, Eisenberg R, et al. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single-centre, retrospective analysis of chart data. Lancet Haematol. 2019;6:e359-e365.
- Coons JC, Albert L, Bejjani A, Iasella CJ. Effectiveness and safety of direct oral anticoagulants versus warfarin in obese patients with acute venous thromboembolism. Pharmacotherapy. 2020;40:204-210. Erratum in: Pharmacotherapy. 2020;40:718.