Patients with various malignancies are at much higher risk for complications, hospitalization, and death from COVID-19 than patients without cancer.1 Patients with cancer have a nearly 2-fold risk for hospitalization and intensive care unit admission and a 5-fold increase in 30-day mortality as a result of infection with COVID-19.1 This is likely related to the patient’s immunosuppressed status through the nature of their cancer, as is the case with many hematologic malignancies, or because the various treatments for cancer that they are receiving also suppress their immune system.2
For those of us who work in oncology, we see a changing landscape of modalities, vaccine recommendations, and therapeutics that rise and fall with the variant waves that have existed over the past 2 years. This makes it difficult to determine the best care for our patients, as well as to counsel them on what should be the next treatment step. We often find ourselves saying “this is the current recommendation, but we have no clue what will happen in the next month or 6 months.”
To illustrate this fact, on March 29, 2022, after this editorial was written but before it was printed, the Centers for Disease Control and Prevention (CDC) updated its booster recommendations to “allow certain immunocompromised individuals and people over the age of 50 who received an initial booster dose at least 4 months ago to be eligible for another mRNA booster to increase their protection against severe disease from COVID-19. Separately and in addition, based on newly published data, adults who received a primary vaccine and booster dose of Johnson & Johnson’s Janssen COVID-19 vaccine at least 4 months ago may now receive a second booster dose using an mRNA COVID-19 vaccine.”3
Early in the COVID-19 pandemic, we had no better tools to protect our patients with cancer than very strict lifestyle precautions, including masking and isolation. With the advent of the COVID-19 vaccine, there was hope that we could provide some protection to our patients; however, a long history of vaccination of patients after a hematopoietic stem-cell transplant (SCT) and of other immunosuppressed patients, suggested that these patients would likely not have the same degree of response as patients with intact or fully functional immune systems.
Follow-up studies have continued to confirm that patients with cancer had blunted and heterogeneous antibody responses to full courses of mRNA vaccines.4-8 In one German study, negative seroconversion occurred mostly in patients with lymphoid malignancies, most of whom were receiving active therapy.4 This was especially true in patients with hematologic malignancies or in patients who were receiving B-cell depleting therapies, including Bruton tyrosine kinase inhibitors, ruxolitinib, venetoclax, or anti-CD20 antibody therapies.5 Another observational study showed that the opposite was true, in that the majority of patients with acute myeloid leukemia or myelodysplastic syndrome seroconverted with subsequent dosing of the COVID-19 vaccines, despite the use of steroids or immunosuppressant drugs.6
At that point, testing for antibody response was not recommended for our patient population, because antibody response did not adequately describe the possible benefits of the testing, and did not assess the potential T-cell response that may still be adequate to provide a survival benefit in patients who have an intact cellular immunity response.7 In November 2021, a study of cellular responses to COVID-19 vaccination, specifically in the multiple myeloma population, further demonstrated that even T-cell response was blunted and variable after vaccination.8 The combination of these studies further supplements the idea that additional prophylactic measures should be considered for high-risk patients for whom vaccination may not result in sufficient protection against COVID-19 infection.9
When combining these data with the changing recommendations from the CDC,10 as more data have emerged, it has become difficult for patients and practitioners alike to keep up with how many doses of the COVID-19 vaccine to administer, and when to do so. Initially, the mRNA vaccines were a 2-dose series for all patients. Data have emerged that suggested that a 3-dose primary series (not to be confused with a booster in the general population) provides better response rates than a 2-dose series in immunocompromised patients, including patients with solid tumors and those receiving immunosuppressants.11,12
Longer-term data initially suggested that protection from the COVID-19 vaccine wanes approximately 5 to 6 months after the completion of the initial series, but information from the CDC has now shown that the booster shots remain highly effective against moderate and severe COVID-19 disease for approximately 2 months after a third dose.13 During the time that the Omicron variant of COVID-19 predominated, the vaccine effectiveness rate was 87% in patients who had received a booster 2 months earlier, but that rate dropped to 66% at 4 to 5 months after receiving the vaccination. Therefore, the vaccine’s effectiveness against hospitalization fell from 91% at 2 months to 78% by the fourth month.13
This reduction in hospitalization has prompted discussions among various cancer centers, cancer organizations, and the CDC regarding additional doses (now 4 doses, or a booster) for immunosuppressed patients. Again, this is not to be confused with a second booster, as we often hear our patients refer to it. It has also added to the confusion that a booster was recommended first at 6 months after the completion of the primary vaccination series, and was then shortened to 5 months after the completion of the series. If this information is difficult for us as healthcare professionals to keep track of, imagine how confusing it is for patients.
Now, as the Omicron variant wave has subsided, many patients are coming up on 5 months past their true boosters, and they may be asking whether another booster is needed. Again, we return to the response “We don’t know yet.” If operationalizing and administering COVID-19 vaccines wasn’t hard enough, the new benefit of pre-exposure prophylaxis with tixagevimab plus cilgavimab (Evusheld) for COVID-19 hit oncology centers hard and fast in December 2021.14
The efficacy of this medication was studied in 2 phase 3 clinical trials, PROVENT and STORM CHASER.14 In the PROVENT trial, tixagevimab plus cilgavimab reduced symptomatic COVID-19 cases by 77% compared with placebo, with no severe symptoms. It is important to note that the total number of actual cases in both study arms was very low. In STORM CHASER, there was no difference in symptomatic cases of COVID-19 before day 30 between the patients who received tixagevimab plus cilgavimab and who received placebo. Thereafter, there was a near 50% reduction of symptomatic COVID-19 in patients who received tixagevimab plus cilgavimab versus those who received placebo, which established the role of the combination in pre-exposure prophylaxis and further emphasized that the combination was not indicated for postexposure prophylaxis.14
One of the biggest downfalls of these clinical trials is that tixagevimab plus cilgavimab was studied primarily during the Delta variant of COVID-19.14 Although the combination retained some efficacy against the Omicron variant, which was predominant at the time of the release of this combination medication, it was not nearly as effective as it could have been. Despite this, any benefit we can provide to our patients in reducing mortality or hospitalization is desirable, especially given the very minimal side-effect profile of tixagevimab plus cilgavimab.14
On the release of tixagevimab plus cilgavimab, many patients with cancer were clamoring for something that could provide the humoral immunity that their own bodies could not produce because of their disease and/or the treatment they were receiving. Although the drug combination was provided free of charge through government purchasing and distribution, it did so with its own operational nightmares. States were given allotments by the federal government to manage and develop different ways of distributing the medication to their healthcare facilities and citizens, with many relying entirely on their healthcare facilities to figure out the process.
In addition, although the tixagevimab plus cilgavimab drug combination was cost-free, its administration might not have been cost-free, and/or there was still pushback from insurers on payments associated with its administration, although the drug was technically covered by the Centers for Medicare & Medicaid Services (CMS), similar to the COVID-19 vaccines. Many pharmacists were fighting battles with prior authorizations for a drug combination that should not have required it. In addition, there was a very limited supply of the medication at first, and many healthcare facilities had to create tiering processes to determine who needed the medication the most, until its supplies increased.
In the state of Idaho, for example, where one of us is practicing, the entire state’s initial supply of tixagevimab plus cilgavimab was a total of 240 doses. Idaho’s Department of Health and Welfare reached out to healthcare facilities (approximately 1-2 weeks before the drug’s release, which was the week before Christmas 2021) to see who could come up with the most efficient process for getting the medication to patients.
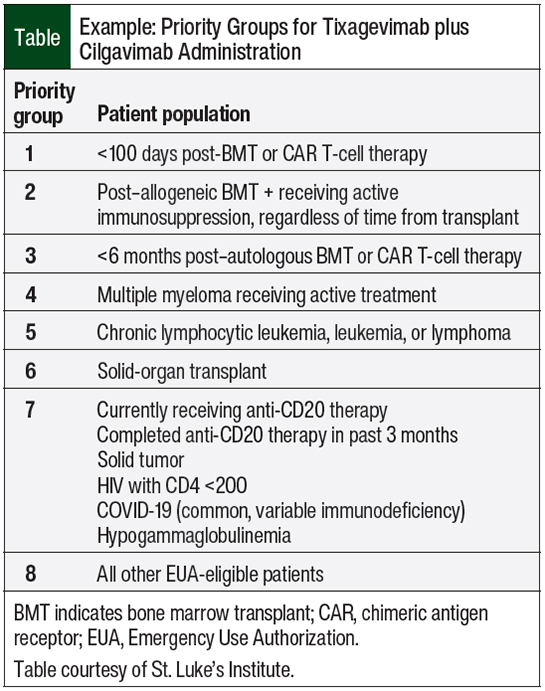
At St. Luke’s Health System, Idaho’s largest oncology care provider, we developed a tiering system to determine who should receive the first supplies of the medication (Table). Based on the National Comprehensive Cancer Network’s recommendations on COVID-19 vaccination and pre-exposure prophylaxis,15 the only patients who could not immediately receive vaccines were those within the 3 months after receiving hematopoietic SCT or cellular therapy, so they became the highest priority tier for receiving prophylaxis with tixagevimab plus cilgavimab.
This tier was followed by patients who had received an allogeneic SCT beyond this period and were still receiving immunosuppression for the prevention or treatment of graft-versus-host disease. The third tier included patients who received an autologous SCT within 6 months, because patients who received the vaccine at 3 months were unlikely to have a good response, based on previously noted data.4-9 Beyond these tiers, there was a lot of discussion among our providers as to the next tiering group. Based on data from the study by Aleman and colleagues8 and personal input from our oncologists, noting that we were seeing disproportionate deaths in patients with multiple myeloma and COVID-19, we decided that patients with multiple myeloma would be in tier 4. The remaining tiers are outlined in the Table.
Initially, the identification of tiers 1 to 3 was controlled by the blood and marrow transplant/hematology pharmacist in scheduling, including for surge clinics, but as we rolled out to tier 4 and beyond, we relied on providers and pharmacists at each of our infusion centers to help identify, schedule, and administer tixagevimab plus cilgavimab to patients. Many of our pharmacists were certified to administer this medication, similar to vaccines, to help with the workflow. As of the end of January 2022, the supply of this medication had increased to the point where we were able to start the administration to all patients eligible for the emergency use authorization (EUA).
In terms of access to tixagevimab plus cilgavimab, we see another approach in Southern California. The attempt of state personnel to distribute the drug in California was originally done regionally, and after a month was pushed out county-wide. Riverside County, CA, is approximately the size of the state of New Jersey. Although New Jersey is larger than Riverside County by only approximately 150 square miles, these geographical areas have vastly different population amounts.16,17 Riverside County is the fourth most populous county in the state of California and the tenth most populous county in the United States; that said, Riverside County is only approximately 25% of the population that New Jersey boasts.16,17
Based on these facts, Riverside County health professionals were scratching their heads trying to figure out, with relatively no warning, how to find the immunocompromised patients quickly in their county. The county had only 2 comprehensive cancer centers, and for expediency’s sake, it was decided to shunt all the county’s allocation of the drug to those 2 sites initially. Although this tactic sounds very reasonable, looking on a map might have helped, because the 2 sites are only 14 miles apart, in a county that spans almost across the entire region of Southern California.
It is easy to point that out now, but the original limiting factor was that only a “hospital provider” could participate in administering the combination drug, and usually that also meant those hospitals that had already been established with the state or county systems in previous monoclonal antibody operations. The added wrinkle was that tixagevimab plus cilgavimab is an outpatient drug combination (for dual gluteal intramuscular administration one after the other) and is not for inpatient use.
A point of pride was that in the Riverside County example, the county health authorities purposefully reached out to the pharmacists at the 2 large hospitals and the local HMO to join a weekly call to troubleshoot and brainstorm, which was one of the most productive communication channels during the whole process. Because there were no officially sanctioned order sheets for tixagevimab plus cilgavimab, the Comprehensive Cancer Center in Palm Springs, CA, did what many providers have done and created an order sheet of its own (Figure).
The billing updates from CMS were shared via web conference, as well as other tips and tricks, so that it became a catch-all for all things related to tixagevimab plus cilgavimab. Although the initial wave of monoclonal antibody administration was started at the end of December 2021 and continued in January and February 2022, on February 24, 2022, the US Food and Drug Administration (FDA) increased the EUA dose for tixagevimab plus cilgavimab, which made the logistics much more complex.18 Currently, the EUA dosing is 300 mg for tixagevimab and 300 mg for cilgavimab (for a combined total of 600 mg) in the gluteal muscles (an increase from 1.5 mL to the current 3 mL in each gluteal muscle).14,18
One of the added nuances is that the FDA guidance also calls for (as with most monoclonal antibodies) an observation period, in this case for a 60-minute period. Although this is prudent and understandable with intravenous administration, when it comes to intramuscular administration, it begs the question if an observation period may be waived as we gain experience with this combination drug. Neither of us has seen an injection reaction that could be termed a significant allergic response (representing thousands of doses).
Once patients started receiving doses of tixagevimab plus cilgavimab, questions arose about COVID-19 vaccination in the context of administering this medication. Although the EUA fact sheet states that patients should wait 2 weeks after vaccination to get tixagevimab plus cilgavimab,14 there were no data about the reverse. The clinical trials suggested that patients should wait from 6 to 12 months, given the medication’s duration of action, but no data were available to suggest safety or efficacy in this case.14
Oncology hematology pharmacists were trying to come up with their own recommendations on when to give additional vaccine doses after receiving monoclonal antibodies. Some pharmacists, using the original data from the AstraZeneca clinical trials inclusion and exclusion criteria, suggested a period of 1 month, based on the drug’s half-life; others suggested 3 months, based on the FDA’s recommendations regarding other monoclonal antibodies; and still others suggested 6 months or longer, based on what was recommended to study participants.14
In February 2022, the CDC issued new guidelines stating that there was no longer a need to have any waiting period for vaccination after the receipt of a monoclonal antibody, used either as treatment or as pre-exposure prophylaxis.10 The rationale again was that any added benefit is better than a possible delay of vaccination, because no demonstrated increase in risks was seen associated with concomitant administration of the vaccine and the antibody prophylaxis.
As mentioned, on February 24, 2022, the FDA revised the indication of tixagevimab plus cilgavimab to double the dose, given the lower efficacy against the Omicron variant.18 This led to many patients having to get a second dose to gain the full anticipated benefit of the antibody prophylaxis, which created another complication for those of us trying to treat our patients appropriately—from patient education and operational standpoints.
The complexity of the continuing changes of COVID-19, the prophylactic measures, and the treatment measures continue to evolve and present challenges for oncology pharmacists and for other providers to deliver consistent, accurate messaging to patients about how best to protect themselves. The best thing we can do is ensure that our patients know that the landscape is continually evolving, review the current state of the data, and provide recommendations based on that.
We must also make sure that our patients know that what we tell them today may be different from what we recommend tomorrow, and that this information is worth discussing often, as time goes on. It should be standard practice to discuss COVID-19 vaccination status, treatment, and other preventive measures routinely at visits with patients to ensure that they know that we are keeping up with the data, and to ensure everyone’s peace of mind that patients have done everything they can, or want to do, to protect themselves.
References
- Sun L, Surya S, Le AN, et al. Rates of COVID-19–related outcomes in cancer compared with noncancer patients. JNCI Cancer Spectr. 2021;5:pkaa120. doi: 10.1093/jncics/pkaa120.
- Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881-2892.
- Centers for Disease Control and Prevention. CDC recommends additional boosters for certain individuals. March 29, 2022. www.cdc.gov/media/releases/2022/s0328-covid-19-boosters.html. Accessed March 29, 2022.
- Rotterdam J, Thiaucourt M, Schwaab J, et al. Antibody response to vaccination with BNT162b2, mRNA-1273, and ChADOx1 in patients with myeloid and lymphoid neoplasms. Blood. 2021;138(suppl 1):218-219.
- Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:e583-e592.
- Jain AG, Dong NC, Ball S, et al. Responses to Sars-Cov-2 vaccines in patients with myelodysplastic syndrome and acute myeloid leukemia. Blood. 2021;138(suppl 1):217-219.
- Kamboj M. Blunted humoral response after mRNA vaccine in patients with haematological malignancies. Lancet Haematol. 2021;8:e540-e542.
- Aleman A, Upadhyaya B, Tuballes K, et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell. 2021;39:1442-1444.
- Tran S, Truong TH, Narendran A. Evaluation of COVID-19 vaccine response in patients with cancer: an interim analysis. Eur J Cancer. 2021;159:259-274.
- Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated March 2, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed March 24, 2022.
- Shroff RT, Chalasani P, Wei R, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27:2002-2011.
- Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661-662.
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263.
- Fact sheet for healthcare providers: Emergency use authorization for Evusheld (tixagevimab co-packaged with cilgavimab). AstraZeneca; February 2022. www.fda.gov/media/154701/download. Accessed March 24, 2022.
- National Comprehensive Cancer Network. Recommendations of the National Comprehensive Cancer Network (NCCN) Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis. Version 5.0. January 4, 2022.
www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v5-0.pdf?sfvrsn=b483da2b_74. Accessed February 17, 2022. - Riverside County, California. Wikipedia. Updated March 6, 2022. https://en.wikipedia.org/wiki/Riverside_County,_California. Accessed February 28, 2022.
- New Jersey. Wikipedia. https://en.wikipedia.org/wiki/New_Jersey. Accessed March 24, 2022.
- US Food and Drug Administration. FDA authorizes revisions to Evusheld dosing. February 24, 2022. www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing. Accessed February 25, 2022.