Phase 1 clinical trials, including first-in-human studies, are important for identifying the safety, tolerance, and pharmacokinetic and pharmacodynamic properties of an investigational drug.1 Relative to later-phase clinical trials, phase 1 clinical trials have additional challenges related to increased protocol complexity, study populations, and resource needs.2-4 Phase 1 cancer clinical trials often involve multiple arms and rapidly changing dose levels; participants in these studies may be complex because of disease progression or have an increased risk for toxicity from previous treatments and concurrent comorbidities; and study teams may need to dedicate additional resources for procedures, pharmacokinetic assessments, molecular profiling, or biopsies.2-4
Multidisciplinary phase 1 study teams consisting of physicians, nursing, pharmacy, phlebotomy, and others support the needs and increasing complexity of these clinical trials.5,6 An Investigational Drug Service pharmacy routinely supports early-phase clinical trials through regulatory compliance and ensuring the safe preparation and dispensing of investigational drugs.5 Investigational Drug Service pharmacists also have the skills to assist with assessing investigational drug regimens, monitoring for adverse events, and identifying clinically relevant drug–drug interactions.5,6
A comprehensive medication review, such as the resolution of drug–drug interactions, for participants enrolled in phase 1 cancer clinical trials is important and should be conducted before starting treatment with an investigational drug.7 A study by Wisinski and colleagues of phase 1 clinical trials that included patients with cancer showed that 69% of patients had at least 1 drug–drug interaction before enrollment, and 15% of patients’ drug–drug interactions remained unresolved after enrollment, because they were not listed as exclusion criteria in the protocol.8 Because clinical trial protocols do not always contain the information needed to assess appropriately drug–drug interactions without additional resources,9 Investigational Drug Service pharmacists may be able to assist in the review of these interactions.
A recent phase 1 clinical trial program at a cancer center over a 9-month study period identified 446 clinical pharmacy interventions, such as concomitant medication review and clinically relevant drug–drug interactions, and concluded that clinical pharmacists are an untapped resource for phase 1 clinical trials.10 This conclusion is consistent with best practice recommendations from the Hematology/Oncology Pharmacy Association (HOPA), which propose that Investigational Drug Service pharmacists provide medication counseling for patients who receive investigational medications, assess medication adherence, and participate in the reporting of unanticipated problems, such as adverse events.5
Phase 1 clinical trials determine important information about the appropriate dose of the investigational drug; however, there is typically limited knowledge of the toxicity profile of a drug during this period of research. As of April 2020, the Johns Hopkins School of Medicine had 71 active phase 1 cancer clinical trials at the Johns Hopkins Medicine Sidney Kimmel Comprehensive Cancer Center (SKCCC).
Because dedicated pharmacy services for phase 1 clinical trials are limited at the Johns Hopkins Medicine SKCCC embedded within multidisciplinary research teams to support the unique needs of these studies, the purpose of this study was to assess the current phase 1 cancer clinical trial practices at National Cancer Institute (NCI)-designated institutions and to develop a proposed framework to establish an integrated pharmacy practice model at the Johns Hopkins Medicine SKCCC.
Methods
The current Investigational Drug Service pharmacy practices at NCI institutions were queried through a 20-question online national survey. The survey questions focused on clinical pharmacy services for phase 1 cancer clinical trials, and were developed in reference to a 2014 national survey conducted by Khandoobhai and colleagues.11
The 20-question online national survey was sent to a total of 208 recipients, including NCI-designated cancer centers (N = 64; excluding Basic Laboratory Cancer Centers) and NCI-affiliated institutions (NCI Community Oncology Research Program and National Surgical Adjuvant Breast and Bowel Project, Radiation Therapy, Gynecologic Oncology Group; N = 144), using Qualtrics XM (Qualtrics; Provo, UT; Version September 2019), with a 60-day window of completion from October 1, 2019, to December 1, 2019.
Weekly reminders were sent out to the recipients about completion of the survey. The recipients were asked to rate how often a specific phase 1 clinical trial pharmacy service was conducted at their site, based on a 4-point Likert scale of: rarely/never (<10%), sometimes (10%-49%), often (50%-80%), and almost always (>80%). Phase 1 clinical trial pharmacy services were grouped into pretrial implementation support, phase 1 trial implementation support, patient medication profile review, medication therapy management, and miscellaneous support.
To determine what clinical pharmacy services were provided by institutions that maintained a similar number of phase 1 cancer clinical trials conducted at the Johns Hopkins Medicine SKCCC, we did a subgroup analysis for institutions that conducted >40 phase 1 cancer clinical trials or ≤40 phase 1 cancer clinical trials. Through this subgroup analysis, we were also interested in determining whether any differences existed based on the number of phase 1 cancer clinical trials conducted at an institution.
The patients’ demographic information was collected, and 2 optional questions were used to identify the highest priority for improving the Investigational Drug Service services pertaining to phase 1 cancer trials, including the perceived barriers to phase 1 trial pharmacy service implementation, and the strengths and weaknesses of current phase 1 trial cancer programs. Descriptive statistics were used to summarize the results.
Leveraging the survey results, we developed a potential framework for clinical pharmacy services in phase 1 cancer clinical trials to prioritize the protocol’s complexity, monitoring requirements, and the opportunity for clinical pharmacy interventions. To create this framework, we conducted collaborative focus groups in 2019 between the Investigational Drug Service pharmacists and the phase 1 research nurses at the Johns Hopkins Medicine SKCCC, and reviewed the current ambulatory oncology clinical pharmacist workflows as a model for clinical pharmacy services within phase 1 cancer clinical trials. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Results
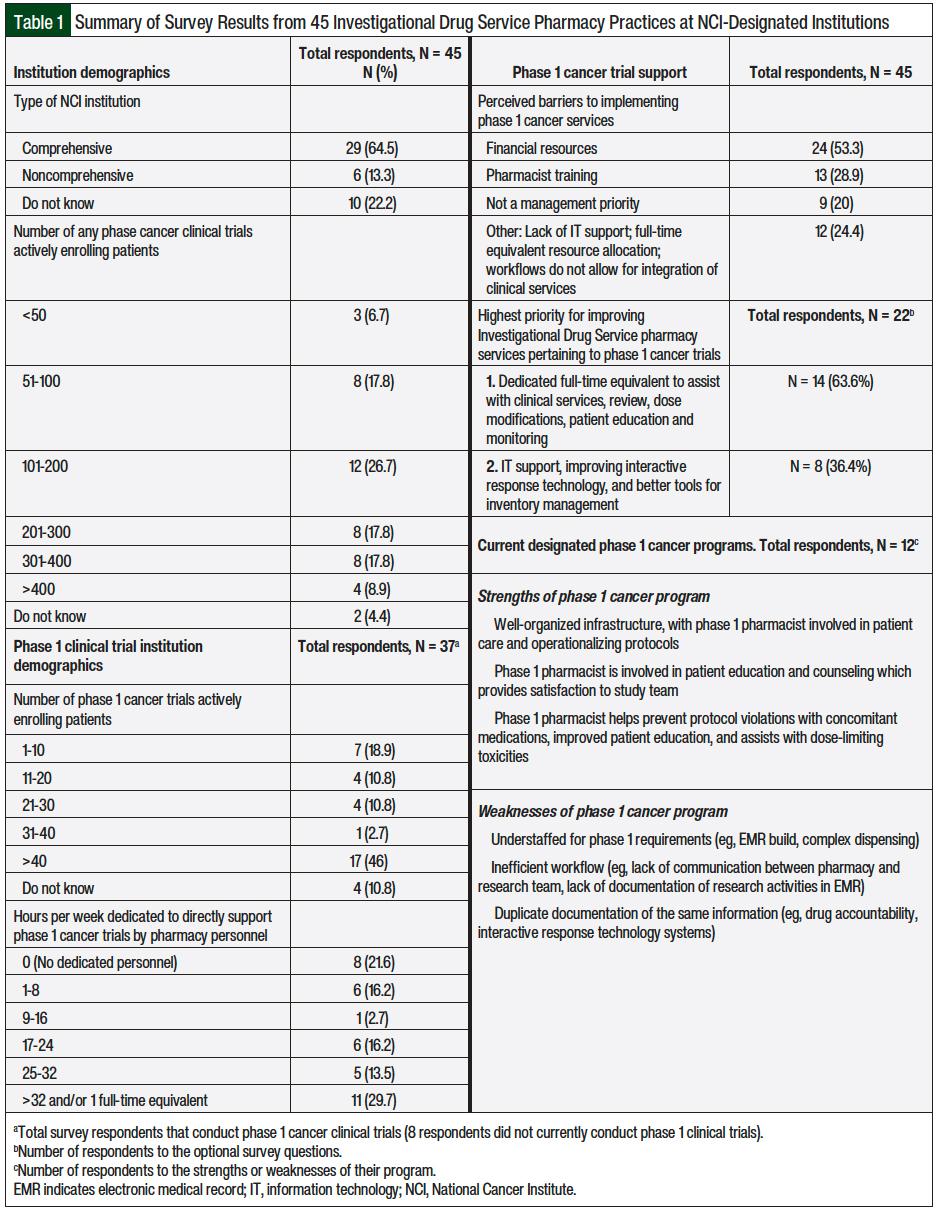
Of the 208 e-mailed surveys, 45 recipients responded, for an overall national survey response rate of 22%. Table 1 summarizes the study results. The total responses included institutions that did not currently conduct phase 1 cancer clinical trials (N = 8) and institutions that did not know the number of phase 1 cancer clinical trials conducted at their site (N = 4). Of the 45 responses, 29 were NCI-designated comprehensive cancer centers (64.5%), 6 were NCI-designated noncomprehensive cancer centers (13.3%), and 10 did not know their NCI status (22.2%). There were 32 (71.2%) institutions with >100 active cancer clinical trials, 11 (24.5%) institutions that had ≤100 active cancer clinical trials, and 2 (4.4%) institutions that did not know the number of active cancer clinical trials.
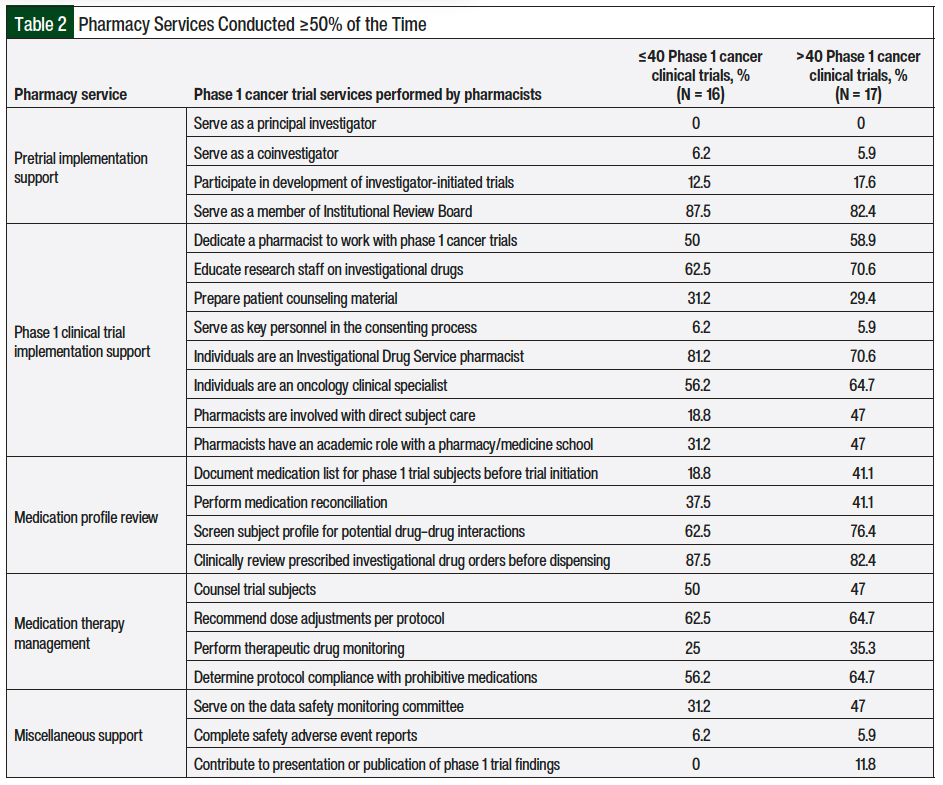
The responses were divided into 2 subgroups for analysis, and institutions were excluded if the number of phase 1 cancer clinical trials was not known (N = 4) or if phase 1 cancer clinical trials were not currently conducted (N = 8). This resulted in a total of 33 institutions for the subgroup analysis, which included sites with >40 active phase 1 cancer clinical trials (N = 17) and institutions with ≤40 active phase 1 cancer clinical trials (N = 16), as outlined in Table 2.
Compared with institutions that have ≤40 phase 1 cancer clinical trials, institutions with >40 phase 1 cancer clinical trials were more likely to have pharmacists involved with direct participant care (47% vs 18.8%, respectively) and to document the medication list for phase 1 trial participants (41.1% vs 18.8%, respectively).
In both subgroups, the designated pharmacy member involved was often or almost always (≥50% of the time) an Investigational Drug Service pharmacist (rather than an oncology clinical pharmacy specialist: 70.6% vs 81.2%, respectively), had Institutional Review Board membership (82.4% vs 87.5%, respectively), reviewed investigational drug orders before dispensing them (82.4% vs 87.5%, respectively), and played a role in educating research staff on investigational drugs (70.6% vs 62.5%, respectively).
For the 3 optional questions, the site-specific responses from our national survey indicated that the highest priorities for improving Investigational Drug Service pharmacy services for phase 1 cancer clinical trials include developing and dedicating at least 1 full-time equivalent to support these services, optimizing information technology support, and improving interactive response technology processes.
For sites that have implemented a phase 1 trial pharmacy program, a total of 12 independent responses were received, and we asked sites to identify the strengths and weaknesses of their program. Of these 12 sites (8 have >40 phase 1 cancer clinical trials, and 10 are comprehensive cancer centers), 5 have pharmacy personnel dedicated to phase 1 clinical trials for >32 hours and/or 1 full-time equivalent.
The additional responsibilities identified through the survey for pharmacists working directly with phase 1 cancer clinical trials included clinical decision support, weekly participation in institutional phase 1 trial meetings, maintaining academic involvement with a school of pharmacy and/or a school of medicine, or serving on a scientific review monitoring committee and/or a data and safety monitoring board.
The perceived barriers to implementing phase 1 clinical pharmacy services included financial resources, pharmacist training, low priority by management, and a lack of information technology support. Other challenges include the staffing model and current workflows (eg, nurses more frequently complete patient medication reconciliations and face-to-face patient visits than pharmacists), and a lack of clarity on daily responsibilities for pharmacists in phase 1 units (overseeing phase 1 studies).
Framework for Clinical Pharmacy Services
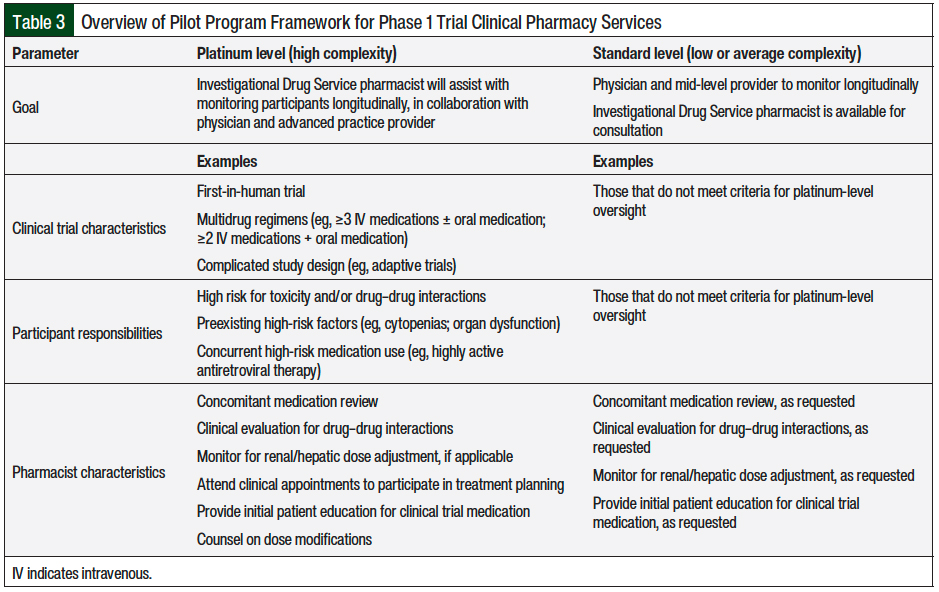
Building on the knowledge obtained from our survey, we developed a framework for the implementation of Investigational Drug Service services to support phase 1 cancer clinical trials as a 3-month pilot program in collaboration with the Investigational Drug Service pharmacists and phase 1 research nurses at the Johns Hopkins Medicine SKCCC. This framework identifies highly complex phase 1 protocols by classifying them as either platinum level (ie, higher complexity) or standard level (ie, lower or average complexity; Table 3).
Examples of factors that would classify a protocol at the platinum level include clinical trials that are first-in-human, adaptive trials (with a potential for multiple amendments); multidrug regimens that have a high risk for adverse events or drug–drug interactions; or patients who have organ dysfunction or are receiving concurrent high-risk medications (Table 3).
For platinum-level regimens, an on-call Investigational Drug Service pharmacist would provide concomitant medication reviews, evaluate for drug–drug interactions, monitor dose adjustments (if applicable) for renal or hepatic function, provide initial patient education for the investigational drug, and counsel patients on dose modifications.
For standard-level regimens, an Investigational Drug Service pharmacist could be available for consultation if requested by a research nurse or a physician to assist with concomitant medication review and drug–drug interactions (Table 3).
Discussion
The landscape of early-phase oncology clinical trials has changed, and the resources needed to support phase 1 cancer clinical trials has increased.2 The key factors that have influenced this change include the increased complexity of study protocols (eg, adaptive clinical trials or expansion cohorts), molecularly targeted agents, and expedited drug approval pathways.2,3
Established pharmacy services can support the increasing complexity of phase 1 cancer clinical trials by assisting with investigational drug regimens, monitoring for adverse events, and identifying clinically relevant drug–drug interactions.
Our study sought to evaluate phase 1 clinical pharmacy services through a national survey and to leverage the survey results to build a framework to implement phase 1 clinical pharmacy services at the Johns Hopkins Medicine SKCCC. With the survey, the study was structured to determine whether any differences existed between phase 1 pharmacy services provided at NCI-designated institutions and institutions with larger volumes of phase 1 clinical trials. Notably, institutions with >40 phase 1 cancer clinical trials were more likely to have pharmacists involved with direct patient care, to document medication lists for phase 1 trial participants, and to serve on a data and safety monitoring committee.
Additional findings from the survey identified perceived barriers for sites to develop a phase 1 trial pharmacy program, which predominantly included financial restrictions for an institution and pharmacist training. The opportunity to be residency trained as an Investigational Drug Service pharmacist was first implemented in 2017. As of 2022, there are 8 Investigational Drugs & Research pharmacy residency training programs (2 accredited by the American Society of Health-System Pharmacists, 1 candidate for accreditation, and 5 precandidates) designed to train pharmacists to support clinical trials research. With a growing number of available residency programs and clinical pharmacists trained in this specialty area, there may be additional opportunities to bridge the training gaps identified in our survey and to further advance pharmacy practice.
We reviewed the current clinical trial pharmacy trends by comparing our national survey results with a national survey conducted by Khandoobhai and colleagues in 2014.11 Compared with the data from the survey by Khandoobhai and colleagues, our survey results showed an increase in pharmacists who document medication lists before starting a clinical trial (23% vs 41.1%, respectively), screening for drug–drug interactions (59% vs 76.4%, respectively), and the education of research staff on investigational drugs (58% vs 70.6%, respectively).11
We leveraged the results of our survey and the findings from the focus groups we conducted to build a framework for a 3-month pilot program to integrate clinical pharmacy services for phase 1 clinical trials within the Johns Hopkins Medicine SKCCC. The phase 1 clinical pharmacy services will focus on platinum-level phase 1 clinical trial protocols, which will be less common than standard-level phase 1 trial protocols (Table 3). The implementation of these services will be consistent with HOPA’s Investigational Drug Service best practice recommendations,5 and will align with pharmacy trends identified from our national survey.
The metrics to be tracked during the implementation of our framework will include the number of concomitant medication reviews conducted, the drug–drug interactions that we identified, and the dose adjustments made (eg, for renal or hepatic function). It will be important to meet with research teams at the conclusion of the 3-month pilot program to determine the processes that worked well and what may be improved.
The current phase 1 cancer program at the Johns Hopkins Medicine SKCCC has highly experienced clinical research managers and nurses, robust institutional research, institutional administrative efforts to reduce regulatory delays, and a large cancer center patient base.2 Research conducted at the Johns Hopkins Medicine SKCCC is integrated throughout the academic institution, and implementing this framework would add to the Johns Hopkins Medicine SKCCC’s successful program by standardizing the approach for highly complex studies and facilitating education and safety for early-phase trial research participants.
Limitations
This study has several limitations. The overall limitations of our survey include the low overall response rate (22%), which can affect the translatability of the findings for current phase 1 pharmacy services. Furthermore, the limited responses that identify the strengths of current phase 1 pharmacy services make it difficult to appreciate fully the details of phase 1 trial pharmacy service programs.
Another limitation is a potential selection bias of the survey recipients, including nonresponses.
In addition, the feasibility and robustness of the proposed framework for integrating clinical pharmacy services into an active phase 1 clinical trial program can only be assessed after the completion of the proposed 3-month pilot program at the Johns Hopkins Medicine SKCCC.
Conclusions
Institutions with increased pharmacist presence within phase 1 cancer trial programs help to preserve physician or research nurse resources that would normally be dedicated to these activities, provide drug expertise, and assist in providing investigational drug education for research team members and patients.
The role of an Investigational Drug Service pharmacist providing clinical care to participants enrolled in phase 1 cancer clinical trials has expanded within institutions that have a need, dedicated resources, and administrative collaboration to support this type of position.
Increased involvement of Investigational Drug Service pharmacists to assess concomitant medications and to conduct comprehensive medication review in highly complex phase 1 trial protocols has the potential to meet the unique needs of these studies, including for clinical trials participants.
Acknowledgment
We wish to acknowledge the research support provided from the phase 1 clinical trial research team at Johns Hopkins Medicine SKCCC, specifically Angela Scardina, Ashley O’Connor, Dr Adrian Murphy, and Dr Nilofer Azad.
Author Disclosure Statement
Dr Rudek received research grants from Celgene Corporation, Cullinan Apollo, RenovoRx, Taiho Pharmaceutical, and Virginia Commonwealth University (parent funding from Gilead), and her spouse is an employee of GlaxoSmithKline and past employee of Novavax. Dr Rudek is a founder of Geminus Therapeutics LLC, serves on its Board of Directors, and holds equity. Under a license agreement between Geminus Therapeutics LLC and the Johns Hopkins University, Dr Rudek and the University are entitled to royalty distributions related to technology not described in this article; Ms Mighty is a Consultant for Visante; Dr Saunders, Dr Murli, Dr Khandoobhai, Dr DeLisa, and Ms Goodrich have no conflicts of interest to report.
References
- Brown JN, Britnell SR, Stivers AP, Cruz JL. Medication safety in clinical trials: role of the pharmacist in optimizing practice, collaboration, and education to reduce errors. Yale J Biol Med. 2017;90:125-133.
- Frankel AE, Flaherty KT, Weiner GJ, et al. Academic cancer center phase I program development. Oncologist. 2017;22:369-374.
- Malik L, Lu D. Increasing complexity in oncology phase I clinical trials. Invest New Drugs. 2019;37:519-523.
- Ivy SP, Siu LL, Garrett-Mayer E, Rubinstein L. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the Clinical Trial Design Task Force of the National Cancer Institute Investigational Drug Steering Committee. Clin Cancer Res. 2010;16:1726-1736.
- Amin SR, Lee JS, Avila JG, et al. HOPA Investigational Drug Service best practice standards. Chicago, IL: Hematology/Oncology Pharmacy Association; 2014. www.hoparx.org/images/hopa/resource-library/guidelines-standards/HOPA16_IDS_Guidelines.reviewed_2018.pdf. Accessed April 26, 2022.
- Aziz MT, Rehman TU, Qureshi S, Andleeb S. Effects of multidisciplinary teams and an integrated follow-up electronic system on clinical pharmacist interventions in a cancer hospital. Int J Clin Pharm. 2017;39:1175-1184.
- McGahey KE, Weiss GJ. Reviewing concomitant medications for participants in oncology clinical trials. Am J Health Syst Pharm. 2017;74:580-586.
- Wisinski KB, Cantu CA, Eickhoff J, et al. Potential cytochrome P-450 drug-drug interactions in adults with metastatic solid tumors and effect on eligibility for phase I clinical trials. Am J Health Syst Pharm. 2015;72:958-965.
- Marcath LA, Coe TD, Hoylman EK, et al. Prevalence of drug-drug interactions in oncology patients enrolled on National Clinical Trials Network oncology clinical trials. BMC Cancer. 2018;18:1155. doi: 10.1186/s12885-018-5076-0.
- Espiritu J, Dai J, Harvey C. Integration of a clinical pharmacist in a phase 1 clinical trial program. J Hematol Oncol Pharm. 2021;11:Abstract PM06.
- Khandoobhai A, Poi M, Kelley K, et al. National survey of comprehensive pharmacy services provided in cancer clinical trials. Am J Health Syst Pharm. 2017;74(suppl 2):S35-S41.