The chronic lymphocytic leukemia (CLL) development landscape is one of the most dynamic areas in oncology clinical research. During the writing of this manuscript, there were new approvals made, and guidelines were updated. Ibrutinib received an expanded approval for the first-line treatment of CLL in March 2016, and venetoclax received accelerated approval for patients with CLL with 17p deletion (del17p) and who have been treated with ≥1 previous therapies.1,2
In February 2014, ibrutinib was approved by the US Food and Drug Administration (FDA) for patients with CLL who had received ≥1 previous treatments, as a result of its superiority over ofatumumab with respect to the progression-free survival (PFS) period (median not reached vs 8.1 months; hazard ratio [HR], 0.22), and the overall survival (OS) rate (84% vs 67%; HR, 0.43).3 In July 2014, the FDA granted accelerated approval of ibrutinib for use in patients with del17p.
Also in July 2014, idelalisib received FDA approval for the treatment of patients with relapsed CLL in combination with rituximab. This approval was based on a 220-patient phase 3 clinical trial that was terminated early because of a significant difference in the PFS rate at 24 weeks (93% vs 46%; P <.001). The accelerated approval allowed the use of the drug in patients with relapsed CLL or with small lymphocytic lymphoma who had received ≥2 previous therapies, based on an overall response rate of 57% and a median time to response of 1.9 months for a variety of indolent non-Hodgkin lymphomas.
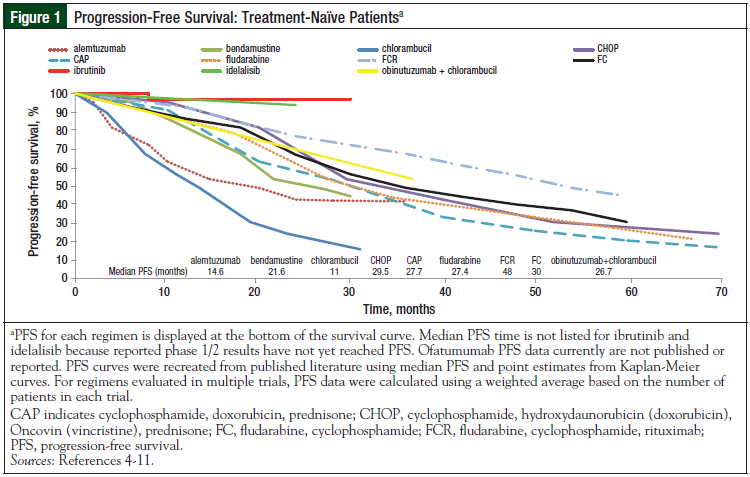
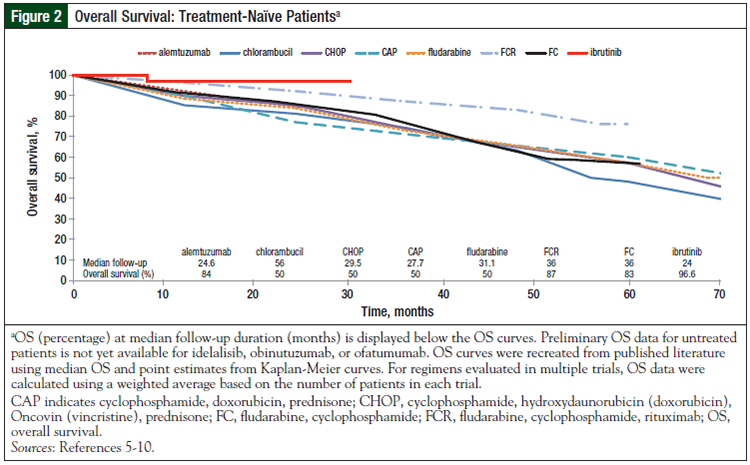
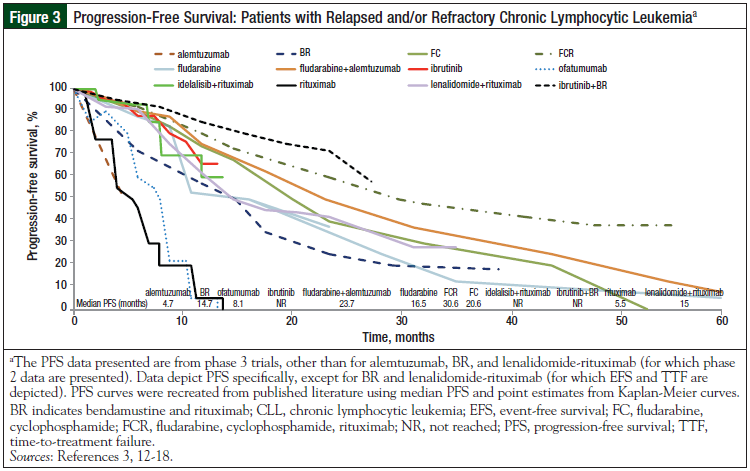
The initial PFS and OS curves (Figures 1-4) show the very promising results of ibrutinib and idelalisib in the untreated and relapsed or refractory patient populations.3-18 Longer follow-up is needed to confirm the durability of these promising results and to determine whether resistance to these agents develops over time.
Ongoing or planned phase 3 clinical trials will expand the role of targeted therapy with ibrutinib and idelalisib in CLL treatment by testing it in combination regimens containing bendamustine, rituximab, cyclophosphamide, obinutuzumab, or ofatumumab in patients with untreated and relapsed or refractory CLL. In addition, entospletinib (GS-9973), a spleen tyrosine kinase inhibitor, is currently being studied in 2 phase 2 clinical trials for relapsed or refractory CLL; one of the trials entails testing it in combination with idelalisib.
There is much excitement surrounding inhibitors of B-cell receptor signaling, including Bruton's tyrosine kinase (BTK) inhibitors (CC-292, ONO-4059, and ACP-196), phosphoinositide 3-kinase (PI3K)-delta inhibitors (idelalisib, IPI-145, and duvelisib), and spleen tyrosine kinase inhibitors. These molecules stop migration and adherence to the CLL microenvironment and promote cellular apoptosis. Two of the most promising agents are venetoclax, which was recently approved for CLL, and IPI-145, an investigational PI3K inhibitor.
Venetoclax was developed through reengineering another BCL-2 inhibitor, navitoclax. Like venetoclax, navitoclax showed therapeutic potential but was hampered by dose-limiting thrombocytopenia caused by BCL-XL inhibition. By improving the selectivity of venetoclax for BCL-2 and decreasing off-target binding at BCL-XL, thrombocytopenia is avoided.14 Both CC-292 and ACP-196 are more potent than ibrutinib and may have a better tolerability profile.
Similar to chronic myelogenous leukemia (CML)—for which second-generation BCR-ABL inhibitors became available after the approval of imatinib and are now part of the CML armamentarium—there is room in the CLL landscape for next-generation BTK inhibitors.
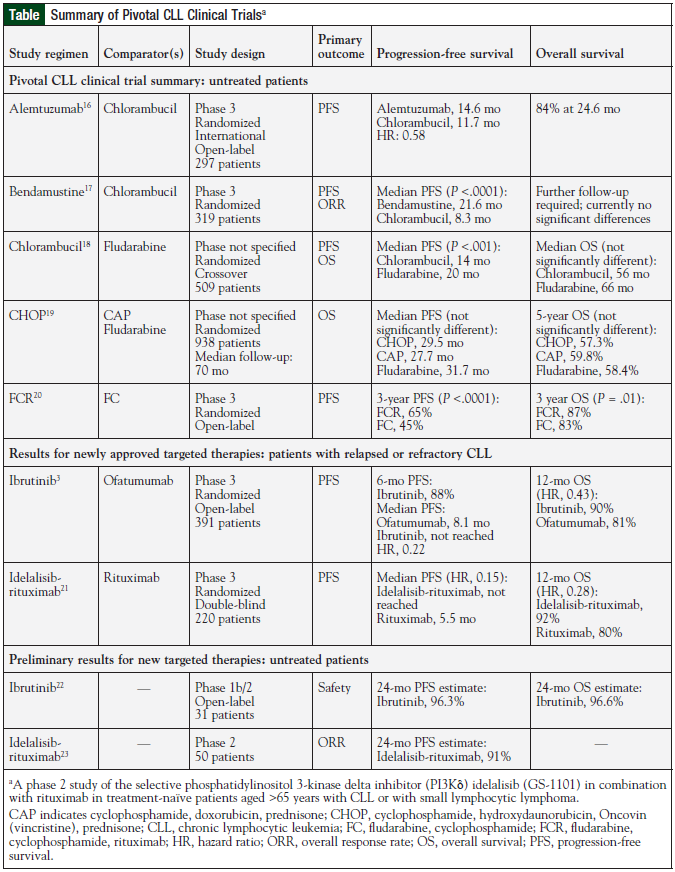
Although the hurdle for activity is now much higher, novel agents that are as or more effective than ibrutinib, idelalisib, and obinutuzumab, with superior safety and tolerability profiles, are needed. Also important is the need for active agents in patients with poor-risk cytogenetics (del17p or TP53 mutation). New drugs in development may have a major impact on the therapeutic landscape of CLL, by increasing the available therapeutic options and by expanding therapeutic combinations and sequencing options (Table).3,16-23
In addition to the targeted agents, checkpoint inhibitors have become breakthrough therapies in solid tumors (eg, lung cancer) and are now being investigated in CLL. A planned phase 1/2 trial of durvalumab is currently in progress. Chimeric antigen receptor T-cells targeting CD19 (CTL019) have induced deep, long-term remissions in patients with relapsed or refractory CLL.10 These promising immunotherapies may be the next treatment strategy for CLL. More research will be needed to identify the best combinations and the patients who are most likely to have deep molecular remissions and responses.
Clinical Trials Landscape
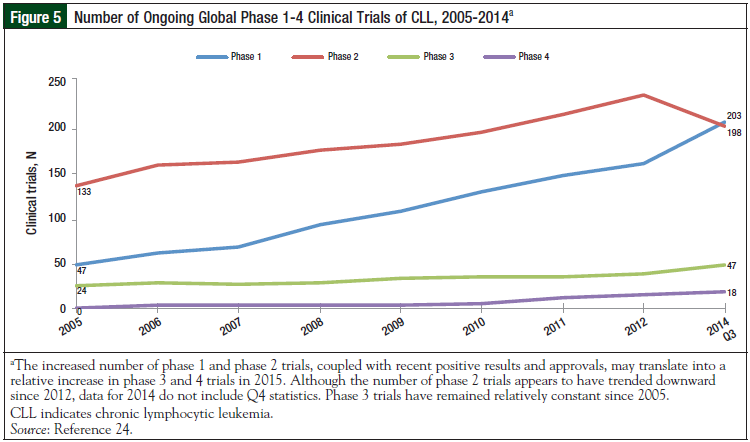
To better evaluate the importance of present evolution in the therapeutic CLL landscape, we examined the number of ongoing, global phase 1 to phase 4 CLL trials, and the cumulative number of completed phase 1 to phase 4 trials. The compound annual growth rates of phase 1, 2, 3, and 4 clinical trials from 2005 to 2014 were 47.42%, 6.98%, 13.69%, and 83.33%, respectively.24 At the time this article was written, there were 116 phase 1, 2, and 3 clinical trials planned or ongoing worldwide in the treatment setting for CLL. This number includes 14 phase 1, 83 phase 1/2 or phase 2, and 19 phase 2/3 or 3 studies. More than half (79) of these trials are in the United States; however, Canada, Europe (France, United Kingdom, Germany), and Asia Pacific (Australia, New Zealand) are also heavily involved.
The United States has the highest number (8) of phase 3 trials, and is also participating in 46 phase 2 trials, and 12 phase 1 trials. Fewer phase 1 trials are ongoing than phase 2 and phase 3 trials, which could indicate that fewer new agents are being investigated, or that many trials may involve testing multiple combinations of novel and established drugs (Figures 5, 6).24
For relapsed or refractory CLL, 100 phase 1 studies, 189 phase 1/2 or phase 2 studies, and 22 phase 2/3 or phase 3 studies are planned or ongoing. Similar to studies of CLL, most of the studies for relapsed or refractory CLL are conducted in the United States and Europe, with the majority being done in the United States (86 phase 1, 87 phase 2, and 13 phase 3). Australia is the most involved country in the Asia Pacific region, conducting 7 phase 3 and 2 phase 2 studies. Each year, the number of ongoing and completed CLL trials continues to increase.
Based on this development landscape and the global epidemiology of untreated and relapsed or refractory CLL, it is likely that CLL research will undergo a drastic change in coming years. Countries such as Ireland and some in the Asia Pacific region (eg, India, Russia, and China) may be of particular interest for new studies, because intense competition for sites and a changing standard of care necessitate relocation to countries where there are few directly competing trials. Opening up sites in smaller, less traditional countries may be beneficial for obtaining more treatment-naïve patient populations and minimizing competition; however, this must be weighed against the added startup costs of using an additional country.
Although CLL incidence is low in Asian countries, large population centers in these regions may provide access to a large number of patients, including those who have not previously participated in clinical trials. Sites in metropolitan areas may be good targets that have low competition. In addition, a movement toward precompetitive collaborations among multiple pharmaceutical companies and academic centers is already in progress, and the scope of these collaborations is likely to increase in the future.
Feasibility of Clinical Trials in a Shifting Therapeutic Landscape
Based on experience and shared intelligence from previous phase 3 trials in CLL, the largest barrier to patient enrollment is the ability and willingness of participating sites and/or countries to use the required concomitant chemotherapy or immunotherapy. With a shifting standard of care in the untreated (bendamustine in combination with rituximab vs fludarabine, cyclophosphamide, and rituximab, ibrutinib, obinutuzumab, and chlorambucil) and the relapsed or refractory settings (venetoclax, ibrutinib, and idelalisib), a careful regional analysis is required before initiating a CLL trial.
Preliminary feasibility analysis is also required. Some countries do not have access, or have only limited access (ie, compassionate use), to the chemotherapeutic agents designated by the study protocol. In recent CLL trials, this difficulty was seen as a result of either eligibility requirements for the previous use of rituximab or bendamustine, or of protocols requiring the inclusion of certain medications in the treatment regimen that might not have been available in a specific region.
These issues may be compounded by different standards of care in certain regions, reimbursement issues, or the regulatory approval status of specific products. Moreover, some investigators may prefer to use standard-of-care regimens or a competing research protocol that they believe will show the same benefit, with reduced toxicity than what is currently being offered. A final barrier to enrollment may be the investigator's perceived ability to enroll the required number of patients. Except for the 10 to 15 countries with the highest prevalence of CLL, the population of patients declines significantly, and, thus, committing to enroll patients at a set rate may not be realistic.12
Although the focus of CLL research has become more personalized with respect to the patient's age, performance status, and cytogenetics, recruitment and early clinical trial matching programs may help determine patient eligibility. Overall, when planning a global CLL trial, strong consideration should be given to the availability of required concomitant medications or previous therapies, and the willingness or ability of the investigator to use those medications. Sites should be supported in their enrollment efforts, and be educated on protocols that may be more CLL subtype-specific.
Conclusion: Clinical Implications of the Evolving Treatment Landscape of CLL
Different clinical development strategies, such as the parallel development of companion diagnostics or the use of precompetitive (or collaborative) clinical trials, should be given consideration for future efforts aimed at managing CLL. Such trials may use adaptive trial designs and Bayesian methods in phase 1/2 settings to assign patients to one of several treatment arms. Each arm could consist of a different agent, combination of agents, or sequence of agents, and comparisons could be made with shared control arms, because patients with different prognostic features may enroll.
This approach could shed light on ways to optimize treatment, conserve resources, and advance our knowledge of CLL treatment faster than the disparate approaches currently being used. With the increased pressure on pharmaceutical companies and contract research organizations to meet enrollment goals in clinical trials, and the increased pressure on investigators and site resources, this strategy may be more efficient for identifying which drugs work best for which patients. With such a dramatic shift in treatment patterns, novel–novel development, including combinations with ibrutinib, idelalisib, or venetoclax, and various sequences, should also be considered.
The potential benefits of collaborative trials can include a control arm that is shared among various sponsors. This would allow companies to save costs and maximize patient enrollment into trials with promising regimens, and not a control regimen (eg, single-agent chlorambucil). This design would also allow for novel–novel combinations, such as investigational monoclonal antibodies, antibody-drug conjugates combined with targeted therapies, or immunotherapy in combination with targeted therapy. With this design, there would be potential to partner early with the FDA on an approval platform, and route to phase 3 trials, as was done with the I-SPY2 (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis 2) trial.11
In addition, within the design, the probability of success could be modeled on an ongoing basis to determine early futility within population subsets, or success and graduation to a phase 3 trial. At present, this trial design is generally limited to phase 2 clinical trials, and phase 3 trials would be designed as traditional, randomized controlled trials. These trials are complex to design and to operate, and require experienced resources to navigate from the concept stage to execution to study completion.
Although the most mature data for ibrutinib and idelalisib show prolonged periods of PFS and OS, the majority of completed trials had relatively small sample sizes. As additional data are collected on these and on forthcoming targeted agents, comparisons to imatinib in CML may become a reality, and would potentially show durable benefit for select patient subsets. In the future, the goals of therapy and objectives in clinical trials will become more rigorous and include minimal residual disease–negative remission, minimal residual disease at selected time points, and durable response.
With optimal sequencing and combination therapy, cure may become the goal in select patients. Patients may soon be able to manage their CLL as a chronic condition, for prolonged periods, with ≥1 orally dosed medications rather than traditional cytotoxic chemotherapy. Some patients may be able to live a normal life span, with a good quality of life. Although this would indicate significant improvement for patients with CLL, it may also necessitate the development of other BTK/PI3K-delta inhibitors for the second- and third-line setting, to combat resistance mechanisms. Further research into these resistance mechanisms will be needed, as well as good companion diagnostics.
Another result likely to manifest from the greatly improved outcomes attained with newer therapies is a decrease in the number of patients with relapsed or refractory CLL who may be available for clinical trial enrollment, which would increase the competition for patients. Enrollment is already a challenge, and if more patients have long-lasting durable response to first-line treatment, then the pool of potential candidates with relapsed or refractory CLL for clinical trials would decrease even further. Furthermore, the small proportion of tumors that do progress on these newly approved first-line regimens are likely to have poor cytogenetic markers, and may develop Richter transformations, disqualifying them from enrollment in a CLL trial. Because of these challenges, collaborative and adaptive trial designs will become increasingly necessary to continue studies and development of CLL therapies.
Author Disclosure Statement
Dr Combest, Dr McAtee, and Dr Reitsma are employees of Pharmaceutical Product Development (PPD), Wilmington, NC. Dr Danford is a Research Fellow at PPD. Dr Andrews and Dr Simmons have no relevant disclosures to report.
References
1. US Food and Drug Administration. All approvals March 2016. www.access data.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Reports.MonthlyApprovalsAll. Accessed May 11, 2016.
2. US Food and Drug Administration. FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm495253.htm. Updated April 11, 2016. Accessed May 11, 2016.
3. Byrd JC, Brown JR, O’Brien S, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213-223.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin’s Lymphomas. Version 2.2016. www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Published 2016. Accessed December 2015.
5. Eichhorst B, Fink AM, Busch R, et al. Frontline chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) shows superior efficacy in comparison to bendamustine (B) and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): final analysis of an international, randomized study of the German CLL Study Group (GCLLSG) (CLL10 Study). Blood. 2014;124:19.
6. Chanan-Khan AAA, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab (BR) in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): first results from a randomized, double-blind, placebo-controlled, phase III study. J Clin Oncol. 2015;33(Suppl):Abstract LBA7005.
7. Seymour JF, Davids MS, Pagel JM, et al. ABT-199 (GDC-0199) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): high complete response rate and durable disease control. J Clin Oncol. 2014;32:Abstract 7015.
8. Dreger P, Schetelig J, Andersen N, et al; European Research Initiative on CLL (ERIC) and the European Society for Blood and Marrow Transplantation (EBMT). Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124:3841-3849.
9. Sanford DS, Wierda WG, Burger JA, et al. Three newly approved drugs for chronic lymphocytic leukemia: incorporating ibrutinib, idelalisib, and obinutuzumab into clinical practice. Clin Lymphoma Myeloma Leuk. 2015;15:385-391.
10. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139.
11. Printz C. I-SPY2 trial yields first results on combination therapy for triple-negative breast cancer. Cancer. 2014;120:773.
12. Leukaemia—estimated incidence and prevalence, adult population: both sexes. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/old/summary_table_site_prev.asp?selection=12280&title=Leukaemia&sex=0&africa=1&america=2&asia=3&europe=4&oceania=5&build=6&window=1&sort=0&submit=%C2%A0Execute%C2%A0. 2012. Accessed January 2015.
13. Goede V, Fischer K, Humphrey K, et al. Obinutuzumab (GA101) plus chlorambucil (Clb) or rituximab (R) plus clb versus clb alone in patients with chronic lymphocytic leukemia (CLL) and preexisting medical conditions (comorbidities): final stage 1 results of the CLL11 (BO21004) phase III trial. J Clin Oncol. 2013;31(Suppl 15):Abstract 7004.
14. Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202-208.
15. Ipsos proprietary prescribing database, Q3 2014. Accessed January 2015.
16. Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616-5623.
17. Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378-4384.
18. Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750-1757.
19. Leporrier M, Chevret S, Cazin B, et al; French Cooperative Group on Chronic Lymphocytic Leukemia. Randomized comparison of fludarabine, CAP, and CHOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;9:2319-2325.
20. Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164-1174.
21. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997-1007.
22. O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48-58.
23. O’Brien SM, Lamanna N, Kipps TJ, et al. A phase II study of the selective phosphatidylinositol 3-kinase delta inhibitor (PI3Kδ) idelalisib (GS-1101) in combination with rituximab (R) in treatment-naïve patients (pts) >65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) [Abstract]. http://meetinglibrary.asco.org/content/117245-132. Accessed January 2015.
24. Citeline: TrialTrove. https://ppd.citeline.com/default.asp. Accessed January 2015.