Patients undergoing hematopoietic stem-cell transplant (HSCT) are at high risk for vaccine-preventable infections as a result of the loss of immunity from previous vaccinations.1-8 Varying degrees of impairment of innate and adaptive immunity after HSCT may delay immune reconstitution for up to 1 year or longer. Additional delays in immune reconstitution have been seen in patients with graft-versus-host disease (GVHD).6,9 Therefore, patients who undergo HSCT are at an increased risk for infections and often require close follow-up.6,9
Late-phase infections (defined as >100 days after HSCT) are most frequently seen in recipients of allogeneic HSCT who are receiving myeloablative conditioning regimens or who have GVHD that requires ongoing therapy.6,9,10 Patients who undergo HSCT are at a high risk for infections caused by encapsulated bacteria, such as Streptococcus pneumoniae.6,10,11
Invasive pneumococcal infections in particular are a significant cause of morbidity and mortality, with a reported incidence of 5.97 infections per 1000 transplant (autologous and allogeneic) recipients.12 Pneumococcal disease can present months to years after HSCT, with a median time to infection of 9 to 15 months after having HSCT.6,13,14
Immunization is one of the most successful and cost-effective strategies to control and reduce vaccine-preventable diseases.1,6,8 The administration of vaccines after HSCT can reduce the risk for serious, and sometimes fatal, infections in this vulnerable patient population.1,6,8,14,15 The measurement of serologic antibody titers have shown that patients who undergo HSCT achieve and sustain posttransplant immunity through vaccination.1,6,8,14,15
The current guidelines from the Infectious Diseases Society of America,8 the American Society for Transplantation and Cellular Therapy (formerly the American Society for Blood and Marrow Transplantation),6 and the European Group for Blood and Marrow Transplantation14 recognize that immunization through vaccination is a critical strategy in protecting against life-threatening infections, and thus recommend routine revaccination at a fixed-dosing schedule for all patients after HSCT.1 Of note, the exact time of vaccine initiation often varies for each vaccine (recommended initiation ranges between 3 and 12 months, depending on the vaccine), transplant type, and transplant center.
Although immunization adherence is one of the key measures for the prevention of infectious complications after a transplant, adherence to the recommended vaccination series is largely understudied.3-5,16-19 A study by Cooper and colleagues showed that only 7.6% of patients who underwent allogeneic HSCT had appropriate documentation of vaccines at 12 months after the transplant.5 However, the total number of patients included in this study was not provided.5 In another study, Teh and colleagues evaluated 77 patients who had autologous HSCT after the implementation of a dedicated nursing vaccination service; the study reported a 98.5% initiation rate and a 48.4% completion rate of the vaccination series.17
Ariza-Heredia and colleagues assessed vaccination adherence rates after autologous and allogeneic HSCT with respect to the initiation of the vaccine series.18 Between 2010 and 2013, a total of 663 patients who underwent HSCT were included, of whom 251 (38%) patients were identified as fully vaccinated, 161 (24%) were partially vaccinated, and 251 (38%) were not vaccinated at the recommended start time of 6 months after transplant. The reasons for not getting vaccinated were relapsed disease, GVHD, and a lack of vaccination documentation.18
Although these studies provide some insight, the overall adherence to the complete vaccination series in patients who have had autologous or allogeneic HSCT over multiple years has not been studied. Evaluating the overall vaccination adherence rates on a large scale will allow for a better determination of the response rates and for the identification of gaps and barriers to adherence, and may provide insight into possible solutions for improving vaccination rates. Therefore, we conducted a retrospective chart review to assess vaccination adherence rates among recipients of autologous or allogeneic HSCT over a 5-year period.
The primary end point of this study was the completion rate of the post-HSCT vaccination series in patients who met the study inclusion criteria. The secondary end points included the identification of reasons for nonadherence when available, assessment of antibody response based on the hepatitis B titer and the measles, mumps, and rubella (MMR) titer, and the rates of vaccine-preventable breakthrough S pneumoniae infections.
Methods
This study was a single-center, retrospective institutional chart review of adults who had autologous or allogeneic HSCT at Yale New Haven Hospital between January 2010 and September 2015. The minimum follow-up period for each patient was 2 years from the date of transplantation. Patients were excluded if they did not meet the specified follow-up time, transferred care to an outside facility or hospital, were aged <18 years, and/or were lost to follow-up.
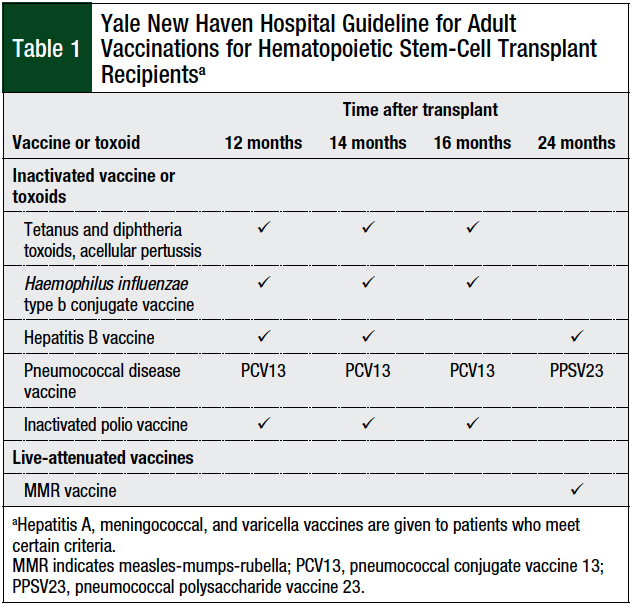
Our transplant center’s post-HSCT vaccination schedule was developed using the international guidelines as a reference, along with expert opinion and experience from our transplant specialists.6,8,14 The vaccination series for autologous and allogeneic HSCT at Yale New Haven Hospital consists of 12-, 14-, 16-, and 24-month immunization time points, and includes Haemophilus influenzae type B conjugate, hepatitis B, inactivated polio vaccine (IPV), tetanus-diphtheria toxoid and acellular pertussis (Tdap), pneumococcal conjugate vaccine (PCV13) and 1 dose of pneumococcal polysaccharide vaccine (PPSV23), and MMR vaccines (Table 1).1,6,8,14
The recommended 3 to 6 months vaccination initiation may not be applicable to patients who had autologous transplant, because of delayed recovery from the transplant and decreased immunity to vaccines if given too early; it may also not be applicable to patients who had allogeneic transplant, because the majority of these patients at our institution do not discontinue immunosuppressive therapy until closer to 12 months after the transplant.
The timing for the initiation of vaccination in these patients is a balance between their response to vaccination, which may be impaired by immunosuppression, and a lack of durability of the response to a vaccine if given early in the posttransplant period.
To ensure consistency between transplant populations, and to facilitate optimal adherence to vaccination guidelines, our institution elected to initiate patients undergoing allogeneic and autologous transplant on a uniform vaccination schedule starting at 12 months after HSCT. For this study, timely initiation of a vaccine series was defined as a vaccine given within 30 days of the recommended time. Delayed initiation was defined as the administration of a vaccine more than 30 days after the recommended time. Per our institutional standard, hepatitis B and MMR titers were collected at 2 months after the completion of the entire vaccination series.
Each patient’s chart was reviewed for baseline characteristics, including sex, age, primary diagnosis, transplantation type, stem-cell type, donor match, chemotherapy conditioning regimen, type of conditioning regimen, type of immunosuppression, and intravenous immunoglobulin (IVIG) administration.
Vaccination administration documentation was queried at Yale New Haven Hospital, unless documentation from the outside physician was available in our charts. Patients who received IVIG within 8 weeks of receiving MMR were excluded from the titer analysis.
We used descriptive statistics to analyze the data in this study.
Results
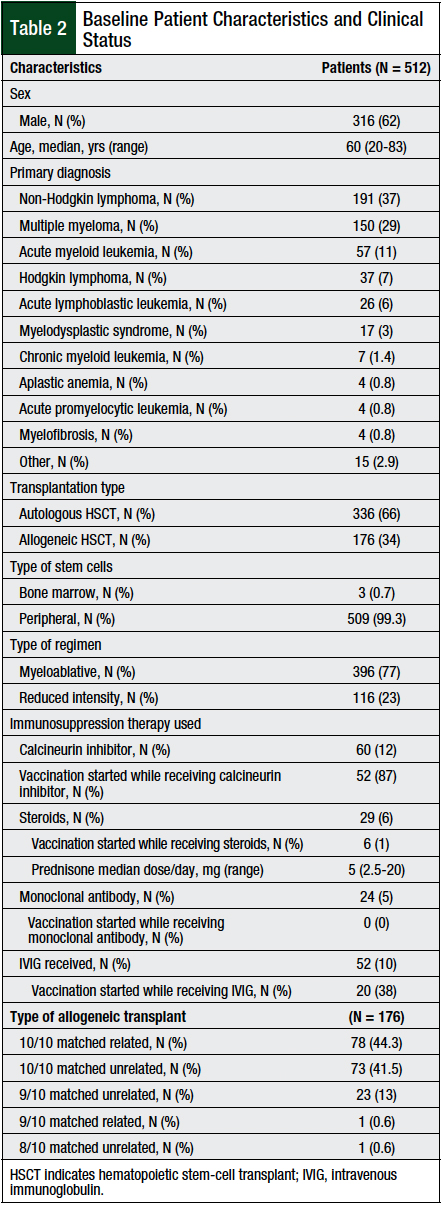
Of the total of 714 patients who were screened, 512 patients were available for analysis. In all, 202 (28%) patients were excluded from the analysis, because they did not meet the specified follow-up period, either as a result of death (N = 117; 58%), being transferred to an outside facility (N = 53; 26%), being younger than age 18 years (N = 25; 12%), or if they were lost to follow-up (N = 7; 3%).
The median age at transplant was 60 years (range, 20-83 years), and 62% of the patients were men. Of the evaluated patients, the majority (66%) of transplants were autologous HSCTs, and the most common (77%) regimen was myeloablative (Table 2).
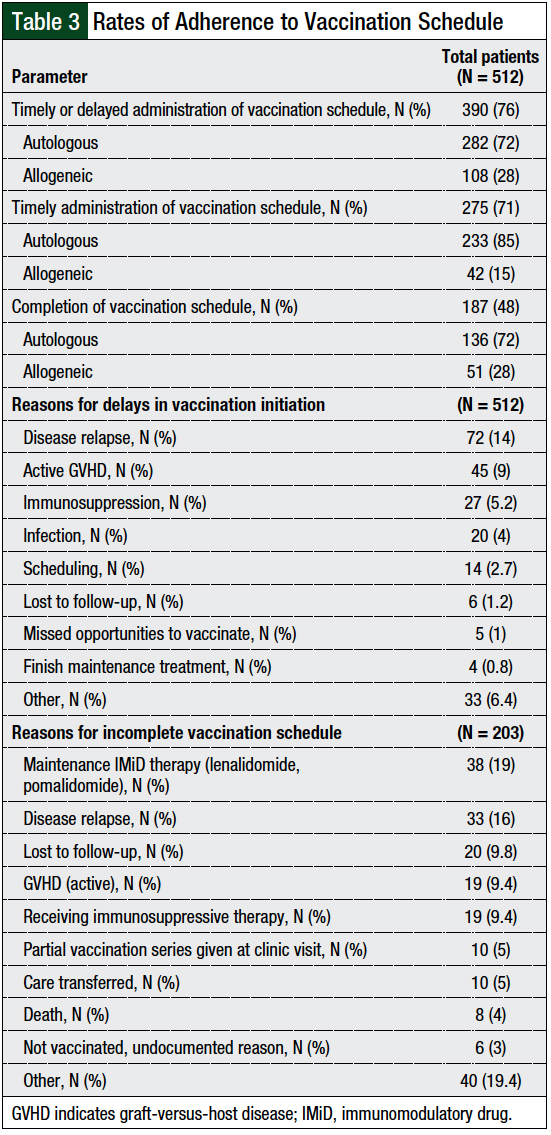
Of the 390 patients who were initiated with the vaccination series, only 187 (48%) patients met the primary outcome of completing the entire vaccination series. The majority (72%) of these patients underwent autologous HSCT.
The most common reasons for not completing the vaccination series in the autologous transplant population (N = 146) included the need for immunomodulatory drug (IMiD) maintenance therapy (24%), disease relapse (20%), and being lost to follow-up (11%). The most common reasons for not completing the vaccination series in the allogeneic transplant population (N = 57) included having GVHD (32%), the need for immunosuppressive therapy (32%), and disease relapse (7%; Table 3).
Of the 203 patients who started vaccination but did not complete the vaccination series, 147 (73%) had autologous transplant and 56 (27%) had allogeneic transplant. Of those receiving autologous transplant, 35 (23%) patients only received 12-month vaccinations, 69 (47%) patients received up to 14-month vaccinations, and 43 (30%) patients received up to 16-month vaccinations. Of the patients who had allogeneic transplant, 7 (12%) patients only received 12-month vaccines, 14 (25%) patients received up to 14-month vaccines, and 35 (63%) patients received up to 16-month vaccines.
When considering all patients receiving autologous and allogeneic transplants, many doses were not given, including 169 doses of hepatitis B vaccine, 140 doses of IPV vaccine, 140 doses of Tdap vaccine, 140 doses of PCV13 vaccine, 129 doses of PPV23 vaccine, and 197 doses of MMR vaccine. Of the 197 doses of MMR vaccine not given, 38 (19%) doses were not given because of active GVHD or because patients were receiving immunosuppressive therapy (Table 3).
Of the 512 patients included in this study, 390 (76%) were initiated on the vaccination series (including timely and delayed vaccine initiation). Of these 390 patients, 275 (71%) initiated the vaccination series on time, at 1-year follow-up, per our institutional guidelines (Table 3). The most common reasons for the noninitiation or delayed initiation of the vaccination series in the autologous transplant population (N = 102) included disease relapse (41%), being unable to meet the scheduled appointments (14%), and unknown reasons (9%). The most common reasons in the allogeneic transplant population (N = 134) were GVHD (33%), the need for immunosuppressive therapy (27%), and disease relapse (22%).
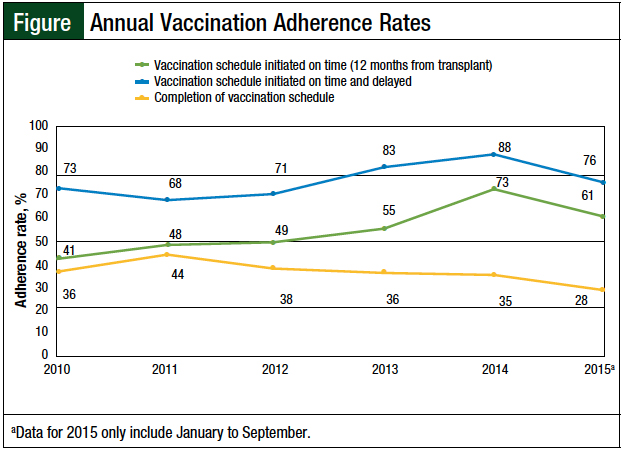
We also analyzed the annual adherence trends, including timely initiation of the vaccination series, as well as delayed initiation and completion of vaccination (Figure). The median adherence rate for the post-HSCT vaccination series initiated at 12 months was 52%; the rate was 75% when the data were combined to include the timely and delayed vaccination schedule patients. The data for the completion of the entire vaccination series revealed a stable median annual adherence rate of 36%.
Of the 187 patients who completed the vaccination series, 36 (19%) had the complete postvaccine titers drawn. The reason for the titers not being drawn was not documented, thus the reasons for nonadherence could not be assessed. A total of 81 patients, including the 36 patients who received the complete postvaccination series, also had antibody titers drawn for hepatitis B and MMR. After at least 1 dose of hepatitis B vaccine, positive titers were noted in 63% of the patients. The MMR vaccine response rates included 79% of patients with a positive titer for measles, 77% of patients with positive titer for mumps, and 97% of patients with positive titer for rubella.
Titer status in the absence of vaccination was also obtained, which demonstrates a retention of immunity after transplantation. All 12 patients undergoing autologous transplant who had posttransplant titers drawn before vaccination revealed retained immunity, including 6 (50%) patients who received measles vaccination, 4 (33%) patients who received mumps vaccination, 6 (50%) patients who received rubella vaccination, and 1 (8%) patient who received hepatitis B vaccination. Similarly, 10 patients who had allogeneic transplant retained some immunity before vaccination, including 1 (10%) patient who received measles vaccination, 2 (20%) patients who received rubella vaccination, and 2 (20%) patients who received hepatitis B vaccination.
Of the patients who received 1 or more doses of pneumococcal vaccination, 8 (2%) patients had a breakthrough S pneumoniae infection. These patients had received a median of 4 doses of pneumococcal vaccine (range, 2-4 doses) before the onset of the breakthrough pneumococcal infection. On follow-up of these 8 patients, 6 patients were receiving steroids at the time of their breakthrough infection, and no patients died as a result of pneumococcal infection.
Discussion
Although international consensus guidelines have published recommendations on vaccination after HSCT,6,8,14 adherence to the posttransplant vaccinations is limited and not well-documented.1-8,16 The few studies that have addressed vaccine adherence conclude that compliance is generally poor, but have not assessed the overall adherence rates in a large sample size of patients undergoing autologous and allogeneic transplant.3-5,16-19 To our knowledge, this is the first retrospective study to assess the overall post-HSCT vaccination adherence rates among patients who had autologous and allogeneic HSCT on a large scale, across multiple years, and before the implementation of an intervention for the optimization of vaccination.
Approximately 71% of the patients in this study received the first set of immunizations on time. Of these 275 patients, 233 (85%) patients had autologous transplant and 42 (15%) patients had allogeneic transplant. Our finding is significantly higher than previous smaller case studies, which reported that only approximately 33% (range, 22%-38%) of patients started the post-HSCT immunizations at the time recommended by their institution.16,18,19 Our results are consistent with previous studies showing that immunization post-HSCT was delayed or withheld most frequently in patients with relapsed disease, with a diagnosis of GVHD, and/or the use of immunosuppressive medications.16,19
A multipart study by Ariza-Heredia and colleagues revealed that although clinician surveys confirmed an understanding of the current post-HSCT immunization guidelines, clinician adherence to the recommendations differed greatly in actual practice, resulting in low vaccine adherence rate.18 These variations were presumably a result of conflicting data on vaccination safety, efficacy, and benefit, coupled with the lack of clear recommendations in complex clinical scenarios, most notably in patients who underwent allogeneic HSCT and had GVHD and received immunosuppressive medication.18
Although most HSCT centers avoid administering live vaccines to patients with GVHD or to patients who are receiving immunosuppressive therapy, several articles have suggested that most live attenuated vaccines may be safely administered in the HSCT population.20-25 Furthermore, the effectiveness of inactivated vaccines in patients with GVHD or in patients who are receiving immunosuppressive therapy is largely unknown, although no evidence exists that GVHD can be caused or worsened by the administration of inactivated vaccines.14 Thus, we believe that the lack of robust literature, as noted above, may explain the low vaccination adherence rate we see in the population of patients undergoing allogeneic HSCT.
In terms of the primary end point of our study, our findings showed that 48% of all patients who started vaccinations completed the entire post-HSCT vaccination series. Adherence with the vaccination series decreased the longer time passed since the transplant. Although Teh and colleagues observed a similar overall adherence rate of approximately 48%,17 a valid correlation between these 2 studies may not be meaningful, because the study by Teh and colleagues only evaluated adherence rates in patients undergoing autologous HSCT.17 In our study, the overall completion of the vaccination series rate was higher (72%) in the autologous HSCT cohort than in the allogeneic HSCT cohort. This is expected, because patients receiving autologous transplant have a lower risk for post-HSCT complications than patients receiving allogeneic transplant, and these complications would affect the timeliness of vaccination.
In our study, 38 (19%) patients with multiple myeloma were receiving lenalidomide as maintenance therapy. The immunosuppressive effects of lenalidomide, a standard-of-care maintenance IMiD in patients with multiple myeloma after HSCT, raised the issue of contraindication to live vaccines, as noted earlier. Thus, the revaccination of patients with multiple myeloma after HSCT is not uniform between cancer centers.
However, a recent study by Palazzo and colleagues of patients with multiple myeloma showed similar response rates to vaccination after transplant in patients who had autologous HSCT and were receiving lenalidomide maintenance treatment and patients who did not receive lenalidomide; they concluded that vaccination with inactivated vaccines in patients receiving lenalidomide was safe and effective.26
Furthermore, Pandit and colleagues studied a cohort of 137 patients who had autologous HSCT and were receiving maintenance treatment with lenalidomide or bortezomib. They concluded that patients who received the MMR or varicella zoster vaccines could safely tolerate the MMR vaccine when given at a median time of 25 months after transplant.27
Based on these findings, and in an effort to increase vaccination adherence and prevent serious infection, we believe that the administration of live vaccines, such as the MMR vaccine, should be reevaluated in patients who are receiving maintenance treatment with an IMiD after transplant.
The majority of studies that evaluate the efficacy of posttransplant vaccination do not measure response by the incidence of breakthrough infection. Rather, response is often measured by serologic antibody titers, which infer seroprotection. In our study, after at least 1 dose of the MMR vaccine and/or the hepatitis B vaccine, the majority of patients responded with a positive titer.
Overall, the number of patients who had complete titers was small, and the reasons for nonadherence were not documented; therefore, our results should be validated in larger studies. In addition, of the 8 patients who received at least 1 dose of the pneumococcal vaccine and had S pneumoniae infection, none had serious complications or fatalities resulting from this infection. However, the serotypes for these breakthrough infections were not available, which, in turn, precluded the assessment of whether these infections were a result of the vaccine or nonvaccine serotypes.
Limitations
This study has several limitations. Our study was restricted to a single transplant center; therefore, our conclusions may not be generalizable to other centers. Currently, we do not have a dedicated vaccination clinic, but follow-up appointments are set up at the time of the patient’s transplant. A multimodal approach and communication between our oncologists, the bone marrow transplant team, and pharmacists are used during post-HSCT follow-up and vaccination administration. Furthermore, when a patient is transferred back to his or her primary care provider, a post-HSCT vaccination schedule is given for follow-up.
At our center, we are unable to determine if the vaccine is administered by a provider outside of our system and/or network unless we are alerted by the provider; this is a potential area of improvement. A proportion of our patients were lost to follow-up and/or transferred their care to an outside facility, and we were unable to capture all the patients who might have received vaccinations at institutions outside of ours. This number, however, was small, thereby demonstrating that our follow-up process is working, which is likely a result of a multimodal team approach.
Furthermore, given the retrospective design, we were limited to the data documented in the electronic medical record (EMR) and were not able to identify consistently the reasons and/or find documentation for vaccination nonadherence in the patient’s chart. Not all hepatitis B and MMR titers were drawn on all patients who completed the entire vaccination series.
An additional limitation is that an updated vaccine guideline from the European Conference on Infections in Leukemia was released after the completion of this analysis.28 One difference between this study and the updated European Conference on Infections in Leukemia guideline is the recommendation of the potential initiation of vaccination in patients undergoing autologous transplant as early as 3 months after the transplant.28 The early initiation of vaccination could improve the rates of adherence, given the increased opportunity to administer vaccines to these patients; however, that was not part of our vaccination guideline at the time of our study. In an effort to standardize our vaccination schedule, we used a 12-month vaccination schedule for all patients. Whether early initiation of vaccination at 3 to 6 months in patients receiving autologous transplant could improve adherence requires further evaluation.
Conclusion
This study adds important data to the limited body of literature on post-HSCT vaccination adherence rates. Vaccination adherence for patients who have had autologous or allogeneic HSCT on a large scale that is expanding over several years and allows for thorough follow-up has not been previously characterized. Despite the availability of international guidelines for post-HSCT revaccination, our study reveals that deviations from the published recommendations often occur.
Based on these data, a multidisciplinary approach is warranted to improve vaccination rates in this setting. This approach may include patient–provider reeducation regarding vaccinations, optimizing the EMR systems for the reassessment of vaccination with alerts at the specified time points, and improving methods of post-HSCT follow-up by adding a dedicated vaccination service or long-term follow-up clinic. Future studies that incorporate these and other interventions to measure and improve post-HSCT vaccination adherence rates are warranted.
Author Disclosure Statement
The authors have no conflicts of interest to report.
References
- Ljungman P, Cordonnier C, Einsele H, et al.Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44:521-526.
- Avigan D, Pirofski LA, Lazarus HM. Vaccination against infectious disease following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:171-183.
- Johnston BL, Conly JM. Immunization for bone marrow transplant recipients. Can J Infect Dis. 2002;13:353-357.
- Carpenter PA, Englund JA. How I vaccinate blood and marrow transplant recipients. Blood. 2016;127:2824-2832.
- Cooper J, Krugh D, Duda J, et al. Improving vaccination of patients pre and post bone marrow transplant. Biol Blood Marrow Transplant. 2012;18(2 suppl):S380. Abstract 481.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238. Erratum in: Biol Blood Marrow Transplant. 2010;16:294.
- Storek J. Immunological reconstitution after hematopoietic cell transplantation–its relation to the contents of the graft. Expert Opin Biol Ther. 2008;8:583-597.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309-318. Erratum in: Clin Infect Dis. 2014;59:144.
- Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33:947-953.
- Hiemenz JW. Management of infections complicating allogeneic hematopoietic stem cell transplantation. Semin Hematol. 2009;46:289-312.
- Srinivasan A, Wang C, Srivastava DK, et al. Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:94-101.
- Engelhard D, Cordonnier C, Shaw PJ, et al; for the Infectious Disease Working Party of the European Bone Marrow Transplantation (IDWP-EBMT). Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol. 2002;117:444-450.
- Molrine DC, Antin JH, Guinan EC, et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood. 2003;101:831-836.
- Ljungman P, Engelhard D, de la Cámara R, et al; for the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Vaccination of stem cell transplant recipients: recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 2005;35:737-746.
- Hilgendorf I, Freund M, Jilg W, et al. Vaccination of allogeneic haematopoietic stem cell transplant recipients: report from the international consensus conference on clinical practice in chronic GVHD. Vaccine. 2011;29:2825-2833.
- Lerchenfeldt SM, Cronin SM, Chandrasekar PH. Vaccination adherence in hematopoietic stem cell transplant patients: a pilot study on the impact of vaccination cards and reminder telephone calls. Transpl Infect Dis. 2013;15:634-638.
- Teh BW, Joyce T, Slavin MA, et al. Impact of a dedicated post-transplant vaccination service at an Australian cancer centre. Bone Marrow Transplant. 2017;52:1681-1683.
- Ariza-Heredia EJ, Gulbis AM, Stolar KR, et al. Vaccination guidelines after hematopoietic stem cell transplantation: practitioners’ knowledge, attitudes, and gap between guidelines and clinical practice. Transpl Infect Dis. 2014;16:878-886.
- Kumar D, Humar A, Plevneshi A, et al. Invasive pneumococcal disease in adult hematopoietic stem cell transplant recipients: a decade of prospective population-based surveillance. Bone Marrow Transplant. 2008;41:743-747.
- Kennedy LB, Li Z, Savani BN, Ljungman P. Measuring immune response to commonly used vaccinations in adult recipients of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23:1614-1621.
- Ljungman P, Cordonnier C, de Bock R, et al. Immunisations after bone marrow transplantation: results of a European survey and recommendations from the infectious diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1995;15:455-460.
- Dykewicz CA. Summary of the Guidelines for Preventing Opportunistic Infections Among Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2001;33:139-144.
- Verolet CM, Posfay-Barbe KM. Live virus vaccines in transplantation: friend or foe? Curr Infect Dis Rep. 2015;17:472.
- L’Huillier AG, Posfay-Barbe KM. Live viral vaccines in transplanted patients. Swiss Med Wkly. 2014;144:w14005.
- Machado CM, de Souza VAUF, Sumita LM, et al. Early measles vaccination in bone marrow transplant recipients. Bone Marrow Transplant. 2005;35:787-791.
- Palazzo M, Shah GL, Copelan O, et al. Revaccination after autologous hematopoietic stem cell transplantation is safe and effective in patients with multiple myeloma receiving lenalidomide maintenance. Biol Blood Marrow Transplant. 2018;24:871-876.
- Pandit A, Leblebjian H, Hammond SP, et al. Safety of live-attenuated measles-mumps-rubella and herpes zoster vaccination in multiple myeloma patients on maintenance lenalidomide or bortezomib after autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:942-945.
- Cordonnier C, Einarsdottir S, Cesaro S, et al; for the European Conference on Infections in Leukaemia group. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19:e200-e212.