The evolution of antiretroviral therapy over the past 2 decades has significantly affected the management of HIV infection and has dramatically increased patients’ life expectancy, giving patients with adequately managed HIV infection life expectancies comparable to those of the general population.1 One study estimated that in 2013 there were 35 million people living with HIV/AIDS, and of those 4 million to 4.5 million people were aged >50 years.2,3 The increase in age of those living with HIV/AIDS is largely attributed to the global uptake and increased use of antiretroviral therapy.2,3
The Department of Health & Human Services (HHS) Panel on Antiretroviral Guidelines for Adults and Adolescents recommends the initiation of antiretroviral therapy in all persons infected with HIV, regardless of their CD4 T-lymphocyte status.4 Historically, nonnucleoside reverse transcriptase inhibitor (NNRTI)-based and protease inhibitor (PI)-based regimens were the regimens of choice for the treatment of HIV infection, but they proved challenging to prescribe, because of their significant side-effect profiles, complex dosing regimens, and potential for drug–drug interactions through the inhibition or induction of the cytochrome (CY) P450 system.5
In the past decade, new agents that are more potent and less toxic have been introduced to the market. The current National Comprehensive Cancer Network (NCCN) guidelines, the HHS antiretroviral guidelines, and a recent study by Olin and colleagues recommend integrase strand transfer inhibitor (INSTI)-based regimens in patients receiving concomitant treatment for HIV and cancer.1,4,6 These regimens consist of 2 nucleoside reverse transcriptase inhibitors combined with an INSTI agent. Alternative options still include NNRTI- and PI-based regimens, which have fallen out of favor, because they are less effective and more toxic than INSTIs.1,4,6
Each class of antiretroviral therapy is associated with different effects on the CYP450 system, most notably the NNRTIs, which act as CYP450 inducers, and the PIs, which act as CYP450 inhibitors.5,7 The properties of these 2 classes of antiretroviral medications have the potential to lead to drug accumulation and subsequent adverse events or decreased drug concentrations and subsequent decreased efficacy if used simultaneously with certain medications, including chemotherapeutic agents.5,7
Breast cancer–related death is among the most common causes of cancer-related deaths in women in the developed and the developing world. Overall, current data do not indicate that a diagnosis of HIV increases the likelihood of breast cancer. However, in some developing countries in southern Africa, the incidence of women with concomitant HIV infection and breast cancer is common.8,9 It is estimated that in these countries, women living with HIV account for 26% of breast cancer cases in women aged <50 years compared with <1% in the rest of the world.9
Phakathi and colleagues have noted that before newer antiretroviral therapy regimens were available, women with HIV who were undergoing surgery, radiation, and chemotherapy for breast cancer were younger at diagnosis, had poorer outcomes, and had more complications than women with breast cancer without HIV.8 In their prospective, observational study of 160 women in South Africa, Phakathi and colleagues reported a mean age of 41 years at diagnosis of breast cancer in patients with HIV compared with 55 years in patients with cancer without HIV; however, they did not find significant differences in surgical outcomes or complications from radiotherapy or chemotherapy.8
The NCCN guidelines for the management of breast cancer recommend surgery with, or without chemotherapy and/or radiation, as primary treatment for patients with breast cancer who are seeking curative intent.10 Similar to antiretroviral agents, various antineoplastic therapies are preferentially metabolized by the CYP450 system and are susceptible to drug–drug interactions.1,5 Chemotherapies, such as cyclophosphamide, doxorubicin, and paclitaxel, are common components of regimens used in the neoadjuvant and adjuvant setting for the treatment of patients with breast cancer and may interact with other substrates, inducers, or inhibitors of the CYP450 system.5,7,10
The data on the management of HIV in patients with breast cancer are exceptionally limited, consisting mostly of case reports and case series or studies conducted in Africa, where the use of breast cancer regimens and HIV regimens may be different from those used in the United States. There is limited information on the extent, severity, and impact of drug interactions between antiretroviral therapy and chemotherapy agents in patients with breast cancer and HIV in the United States.
When treating breast cancer with curative intent, it is possible that dose reductions or delayed doses of chemotherapy will lead to decreased efficacy and worse outcomes for patients. Similarly, the need for holding or discontinuing antiretroviral therapy because of drug–drug interactions can put patients at a significant risk for opportunistic infections or for resistant mutations of HIV. Moreover, it is unknown if newer, INSTI-based regimens may be a safer treatment option in patients who are receiving concomitant chemotherapy compared with PI- or NNRTI-based regimens in this patient population.
The objectives of this study were to evaluate the incidence of grade 3 or 4 adverse events from chemotherapy in patients with breast cancer who are receiving concomitant antiretroviral therapy for HIV, and to examine if adverse events resulting from drug–drug interactions led to changes in chemotherapy or therapy for HIV.
Methods
This single-center, retrospective chart review included patients with breast cancer and HIV who were aged ≥18 years and were receiving chemotherapy with curative intent. The patients were identified through pharmacy informatics reports that identified patients with a diagnosis of breast cancer and a diagnosis of HIV who received treatment at The University of Texas MD Anderson Cancer Center between January 1, 2008, and August 31, 2018. Patients were included if they received at least 1 dose of chemotherapy at MD Anderson Cancer Center. Patients were excluded if they were receiving chemotherapy with a noncurative intent, or if they received chemotherapy at a hospital other than MD Anderson Cancer Center.
We collected the data from the MD Anderson Cancer Center electronic medical records regarding patient demographics, baseline laboratory testing and laboratory testing before each cycle of chemotherapy, and chemotherapy and HIV regimens information. All antiretroviral regimens and chemotherapy regimens were entered into the Clinical Pharmacology powered by ClinicalKey (Elsevier/Gold Standard; Tampa, FL)11 for potential drug interactions, and were reported as “major” or “moderate” drug interactions.
We defined grade 3 and 4 adverse events based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.12 The use of opportunistic infection prophylaxis and of growth factor were based on recommendations from the NCCN guidelines and from the HHS Panel on Opportunistic Infections in Adults and Adolescents with HIV.13,14
All the data reporting is descriptive in nature. The study received Investigational Review Board approval and was granted a waiver of informed consent.
Results
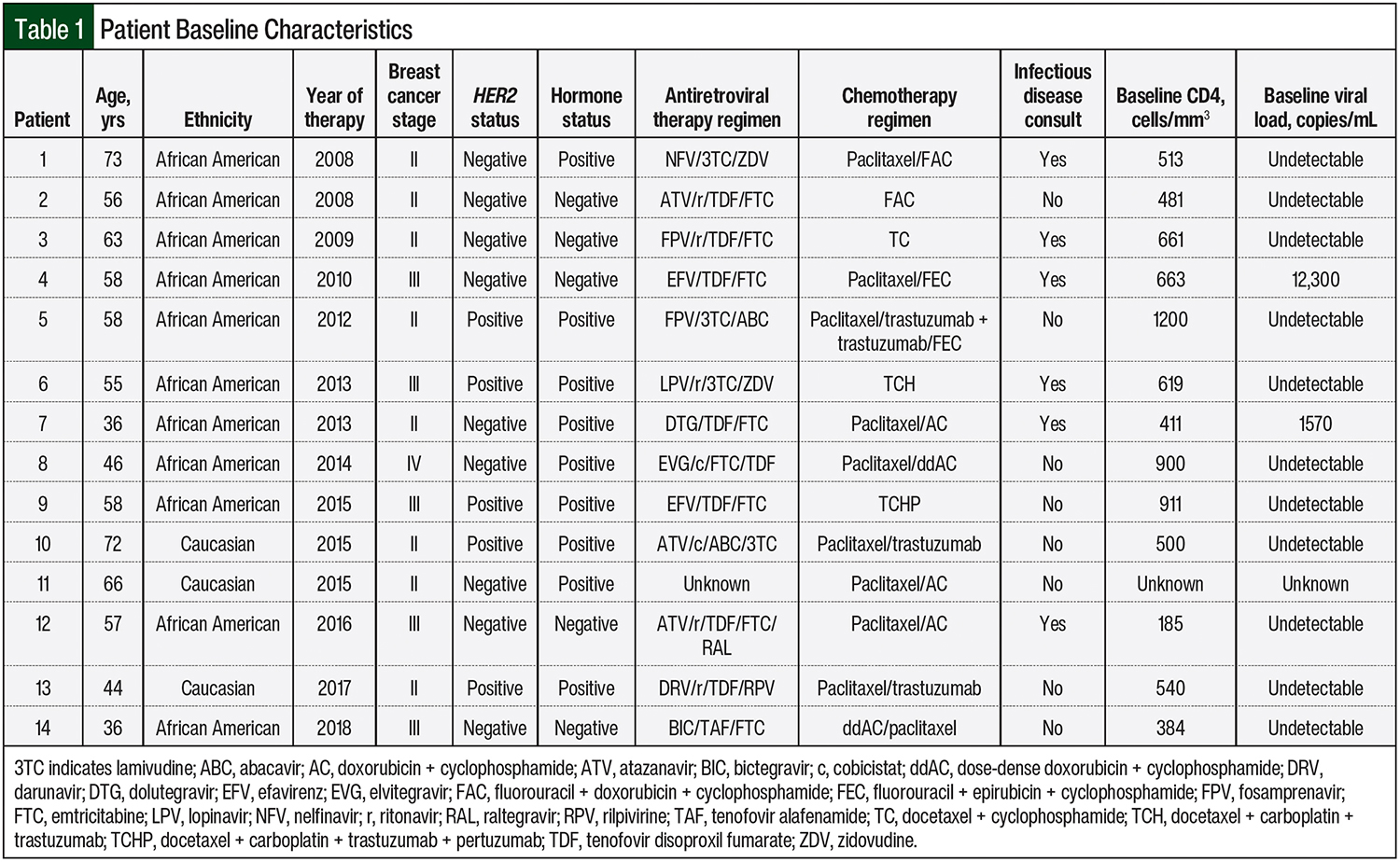
From January 1, 2008, to August 31, 2018, 36 patients were identified at MD Anderson Cancer Center who had a diagnosis of breast cancer and a diagnosis of HIV. After applying the exclusion criteria noted earlier, 11 of the 36 patients did not receive cytotoxic chemotherapy, 6 patients did not receive chemotherapy at MD Anderson Cancer Center, and 5 patients were not receiving chemotherapy with curative intent. These patients were subsequently excluded, leaving 14 patients to be included in this study. The baseline characteristics of the 14 patients are shown in Table 1.
The patients’ mean age was 55 years (range, 36-72 years), and 79% (N = 11) of them were African American. The majority of the patients had human epidermal growth factor receptor 2–negative breast cancer (64.3%; N = 9), and 64.3% (N = 9) of the patients had hormone receptor–positive breast cancer. More than half (57.1%; N = 8) of the patients were receiving treatment for a stage II breast cancer.
The most common chemotherapy regimens used were docetaxel plus cyclophosphamide, fluorouracil plus doxorubicin and cyclophosphamide, fluorouracil plus epirubicin and cyclophosphamide, and weekly paclitaxel, followed by doxorubicin and cyclophosphamide or by dose-dense doxorubicin plus cyclophosphamide.
At baseline, 78% (N = 11) of the patients had undetectable viral loads, and all but 2 patients had CD4 cell counts of >200 cells/mm3; 1 patient had a CD4 count of 185 cells/mm3, and the other patient’s baseline CD4 count was unknown. Of the 14 total patients, 13 had received known antiretroviral therapy regimens that could be assessed, and 88 chemotherapy cycles from the combined 14 patients were assessed.
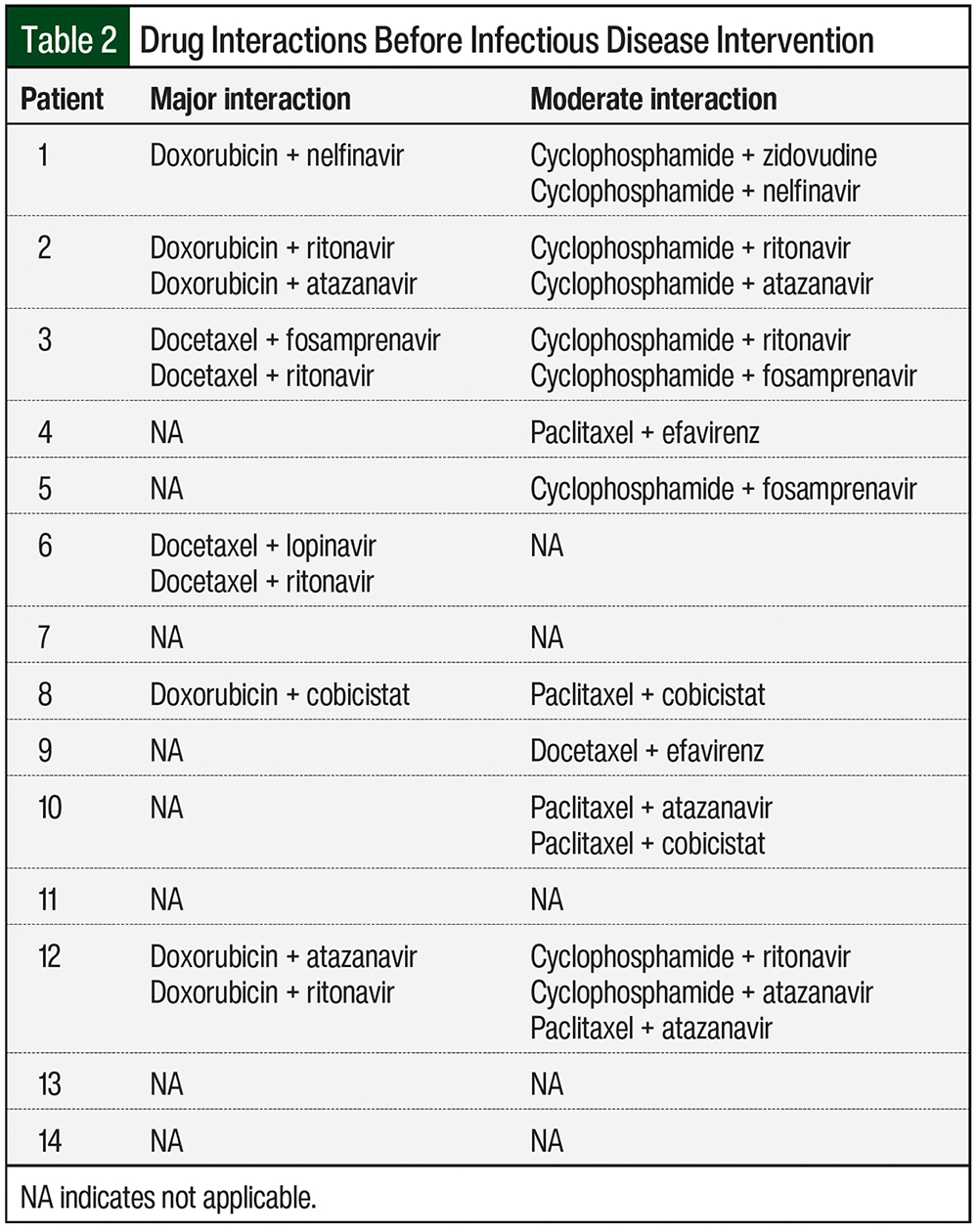
Drug interactions were classified based on their severity, and were defined as “major interaction” when the co-administration of the medications should be avoided, and as “moderate interaction” when the co-administration of the agents should be done with caution. Table 2 lists the major and moderate drug interactions in our 14 patients, before intervention by the Infectious Disease service.
The majority of patients (57.1%; N = 8) were receiving PI-based antiretroviral therapy. This resulted in 10 potential major drug interactions that were identified in 6 patients (Table 2). These major interactions primarily resulted from the concomitant use of docetaxel or doxorubicin and PIs, which could potentially lead to increased concentrations and a subsequent increase in chemotherapy adverse events.
A total of 15 moderate drug interactions were identified in 9 patients (Table 2). The Infectious Disease service was consulted regarding the drug interactions in 6 of the 14 (42.9%) patients. In 2 patients, by adjusting the antiretroviral therapy before the start of chemotherapy, the Infectious Disease service prevented 4 major and 2 moderate interactions.
Patient 6 was initially receiving lopinavir, ritonavir, lamivudine, and zidovudine, but because of concerns for major interaction of lopinavir and ritonavir with docetaxel, the patient was subsequently switched to raltegravir, tenofovir disoproxil fumarate, and emtricitabine, a regimen that was identified as having no interaction with the patient’s chemotherapy regimen.
Patient 12 was initially receiving atazanavir, ritonavir, tenofovir disoproxil fumarate, emtricitabine, and raltegravir. Ritonavir and atazanavir were identified as having major interactions with doxorubicin, so the patient was subsequently switched to treatment with raltegravir, tenofovir disoproxil fumarate, and emtricitabine.
After adjustments to therapy, 6 patients received PI-based therapy, 5 patients received INSTI-based therapy, and 2 patients received NNRTI-based therapy.
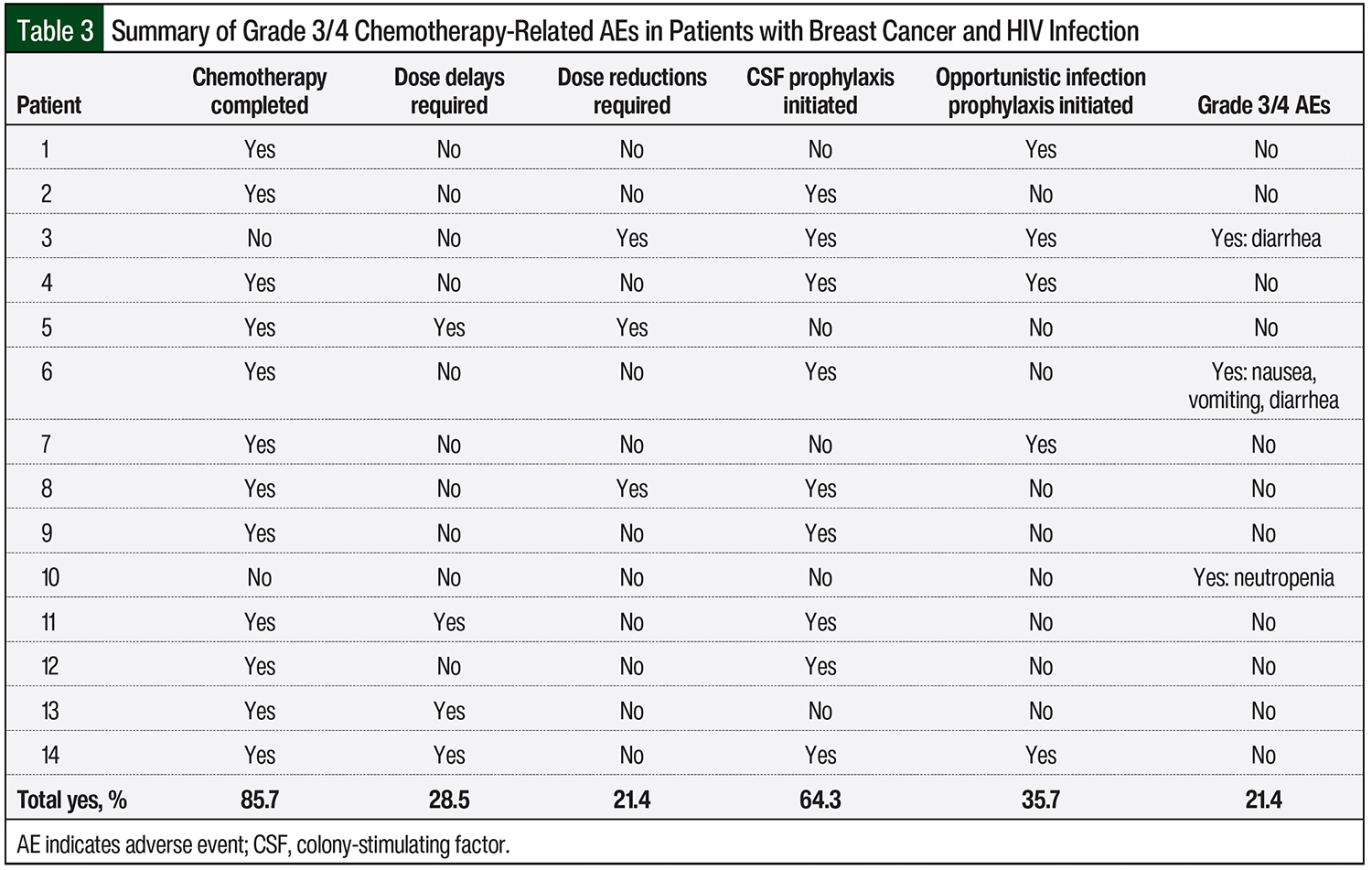
Table 3 summarizes the key study outcomes. Although only 1 patient had a known CD4 count of <200 cells/mm3, 5 of the 14 (35.7%) patients were initially receiving opportunistic infection prophylaxis with sulfamethoxazole plus trimethoprim, azithromycin, or a combination of the 2 treatments (Table 3). A progress note for patient 7 stated that the use of sulfamethoxazole and trimethoprim plus azithromycin resulted from an anticipated decline in blood counts that were secondary to chemotherapy.
There were no reported opportunistic infections in any of the patients during the course of chemotherapy, and no patients required holding, a delay, or the discontinuation of antiretroviral therapy.
Throughout the course of therapy, 12 of 14 (85.7%) patients completed the planned number of chemotherapy cycles; patients 3 and 10 did not complete the planned number of cycles of chemotherapy (Table 3). After the completion of chemotherapy cycles 2 and 3, patient 3 had grade 3 nausea, vomiting, and diarrhea, which reportedly affected the patient’s adherence to antiretroviral therapy. After the completion of chemotherapy cycle 2, patient 10 had febrile neutropenia that required hospitalization. The patient subsequently opted to discontinue the chemotherapy.
Chemotherapy dose reductions were required in 3 of the 14 (21.4%) patients, one of which was a 25% reduction in the initial dosing of paclitaxel because of concern for an increased risk for toxicity with the combination of paclitaxel and cobicistat (Table 3). The patient tolerated treatment well and was eventually titrated to the full dose of paclitaxel at week 9 of therapy. Other dose reductions were unrelated to hematologic adverse events. Dose delays occurred in 4 of 14 (28.5%) patients (Table 3).
Patient 11 was receiving doxorubicin plus cyclophosphamide and required a 1-week dose delay and the addition of colony-stimulating factor (CSF) as a result of neutropenia (absolute neutrophil count [ANC], 1.05/mm3), but resumed therapy and did not require any other dose delays. Other dose delays were unrelated to adverse events and included problems with transportation and trouble with insurance clearance. Grade 3 or 4 adverse events occurred in 3 of 14 (21.4%) patients. Patients 3 and 6 had grade 3 nausea, vomiting, and/or diarrhea, and patient 10 had febrile neutropenia (ANC, 0.38/mm3; Table 3).
Discussion
Based on a study with a small population of patients with breast cancer, Ngidi and colleagues found that the ratio of neutropenic episodes in HIV-infected patients with breast cancer compared with non–HIV-infected patients with breast cancer was 3:1.15 The presence of HIV was determined to be a significant predictor of having neutropenia in this patient population (hazard ratio, 1.76; 95% confidence interval, 1.06-2.92; P = .029). These neutropenic episodes resulted in the need for chemotherapy dose delays, dose reductions, and the administration of CSFs.15
Similarly, in 2004 Bundow and colleagues reported 2 cases of patients with AIDS-related Kaposi sarcoma (but not breast cancer) who were receiving paclitaxel, a common chemotherapy also used for the treatment of breast cancer. Both patients were concurrently being treated with an antiretroviral therapy consisting of a PI.16 The drug interactions between paclitaxel and the PIs led to increases in paclitaxel concentrations and associated adverse events. Throughout the course of therapy, the 2 patients had life-threatening cytopenias that resulted in the need for significant dose reductions to their chemotherapy regimens.16
El-Rayes and colleagues reported about 5 women with HIV who received cytotoxic therapies for breast cancer, including cyclophosphamide, doxorubicin, and 5-fluorouracil. In all these cases, grade 4 neutropenia was documented, and subsequent dose reductions and the addition of CSFs were required for 3 of the women.17 In these 2 reports of multiple cases, the authors concluded that myelosuppression from chemotherapy was more prominent in patients with HIV, and that myelosuppression may be caused by the additive effects of antiretroviral therapy and cytotoxic therapy.16,17
To our knowledge, our study represents one of the few case series in the United States examining the impact of concomitant HIV treatment on the management of patients with breast cancer who are receiving chemotherapy for curative intent.
Although our study was small, our results suggest that the incidence of grade 3 or 4 adverse events in patients receiving concomitant chemotherapy and antiretroviral therapy occurred in a small proportion (21.4%) of this patient population, which differs from the results seen in other studies.15-17 This may be because 9 (64.3%) patients in our study received growth factors. A total of 5 of our patients were receiving chemotherapy regimens, for which primary prophylaxis with CSFs is indicated (such as dose-dense doxorubicin plus cyclophosphamide, docetaxel plus cyclophosphamide, and docetaxel plus carboplatin and trastuzumab), so the incidence of grade 3 or 4 neutropenia would be low in these patients.
However, based on the adverse events reported in previous studies,15-17 it may be worthwhile to monitor patients who are receiving antiretroviral therapy and chemotherapy carefully and to initiate a growth factor at the first sign of lower-than-expected neutrophil counts.
Another promising finding in our study was that 85.7% (N = 12) of our patients completed their planned number of chemotherapy cycles, which is very important when the goal of treatment is a cure. The use of opportunistic infection prophylaxis in these patients is an interesting finding. As noted earlier, only 1 of our patients had a documented baseline CD4 count of <200 cells/mm3 and would, per the Panel on Opportunistic Infections in Adults and Adolescents with HIV guideline recommendations, require prophylaxis for pneumocystis jiroveci; yet 35.7% (N = 5) of the patients were receiving prophylaxis with sulfamethoxazole and trimethoprim.14
This practice may not be prudent, because it may put the patient at risk for increased drug interactions or additive adverse events (such as renal or hepatic dysfunction and cytopenias) when prophylaxis is not necessary. Although these patients were receiving chemotherapy for the treatment of their malignancy, recommendations for opportunistic infection prophylaxis should still be followed, reserving prophylaxis for patients with CD4 counts below the acceptable threshold.
By consulting our Infectious Disease service, the initial antiretroviral regimens were adjusted to decrease the number of drug interactions before the start of chemotherapy. In patients 6 and 12 (Table 1), the regimens were adjusted from PI-based regimens to INSTI-based regimens. Adjustments to these 2 regimens prevented 6 drug interactions. This finding supports the important role for the Infectious Disease service or an HIV specialist in the management of patients with HIV before beginning a chemotherapy regimen, as the NCCN guidelines for the management of cancer in patients with HIV recommend.6 In addition, INSTI-based regimens should be strongly considered in this patient population, because they decrease the risk for significant drug interactions.
Limitations
The limitations to our study include its retrospective nature, the extended period of time over which patients were included, and the very small patient population.
This study’s retrospective nature led to reliance on charted notes and patient-reported events. Many patients had their HIV managed at outside institutions, so the regimens, CD4 counts, and supportive medications were patient-reported and were not followed or adjusted by MD Anderson Cancer Center physicians.
In addition, the 10-year time period might have affected the study results, because the management of patients with breast cancer and HIV changed between 2008 and 2018.
Conclusion
Although this study was small, it provides information about certain patterns in patients with breast cancer and HIV who are receiving concomitant antiretroviral therapy and chemotherapy. These patterns include the safe co-administration of chemotherapy and antiretroviral therapy, the need for close monitoring of patients, caution in the overuse of opportunistic infection prophylaxis medications in patients with CD4 counts of >200 cells/mm3, and utilizing infectious disease consultations to optimize antiretroviral therapy regimens and reduce potential drug–drug interactions.
Larger reviews should be conducted that may prove beneficial to healthcare teams who are managing patients with breast cancer and HIV who are receiving antiretroviral therapy and concomitant chemotherapy.
Author Disclosure Statement
Dr Joseph, Dr Foolad, Dr Patel, and Dr Kaushik have no conflicts of interest to report. Dr Karuturi is a Consultant to Pfizer.
References
- Olin JL, Klibanov O, Chan A, Spooner LM. Managing pharmacotherapy in people living with HIV and concomitant malignancy. Ann Pharmacother. 2019;53:812-832.
- McCormack VA, Febvey-Combes O, Ginsburg O, dos-Santos-Silva I. Breast cancer in women living with HIV: a first global estimate. Int J Cancer. 2018;143:2732-2740.
- Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS. 2014;28(suppl 4):S453-S459.
- Department of Health & Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed March 29, 2022.
- Berretta M, Caraglia M, Martellotta F, et al. Drug–drug interactions based on pharmacogenetic profile between highly active antiretroviral therapy and antiblastic chemotherapy in cancer patients with HIV infection. Front Pharmacol. 2016;7:71. doi: 10.3389/fphar.2016.00071.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Cancer in people with HIV. Version 2.2022. February 3, 2022. www.nccn.org/professionals/physician_gls/pdf/hiv.pdf. Accessed March 28, 2022.
- Flepisi BT, Bouic P, Sissolak G, Rosenkranz B. Drug–drug interactions in HIV positive cancer patients. Biomed Pharmacother. 2014;68:665-677.
- Phakathi BP, Basson G, Karusseit VOL, et al. The effect of HIV infection on the surgical, chemo- and radiotherapy management of breast cancer. A prospective cohort study. Int J Surg. 2016;34:109-115.
- Brandão M, Bruzzone M, Franzoi MA, et al. Impact of HIV infection on baseline characteristics and survival of women with breast cancer. AIDS. 2021;35:605-618.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer. Version 2.2022. December 20, 2021. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed March 29, 2022.
- Clinical Pharmacology powered by ClinicalKey. Tampa, FL: Elsevier. www.clinicalkey.com/pharmacology/login. Accessed September 10, 2020. [Subscription required to access.]
- US Department of Health & Human Services. Common Terminology Criteria for Adverse Events. Version 4.0. May 28, 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed March 28, 2022.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Hematopoietic growth factors. Version 1.2022. December 22, 2021. www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed March 29, 2022.
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Updated February 17, 2022. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection. Accessed April 4, 2022.
- Ngidi S, Magula N, Sartorius B, et al. Incidence of chemotherapy-induced neutropenia in HIV-infected and uninfected patients with breast cancer receiving neoadjuvant chemotherapy. S Afr Med J. 2017;107:595-601.
- Bundow D, Aboulafia DM. Potential drug interaction with paclitaxel and highly active antiretroviral therapy in two patients with AIDS-associated Kaposi sarcoma. Am J Clin Oncol. 2004;27:81-84.
- El-Rayes BF, Berenji K, Schuman P, Philip PA. Breast cancer in women with human immunodeficiency virus infection: implications for diagnosis and therapy. Breast Cancer Res Treat. 2002;76:111-116. Erratum in: Breast Cancer Res Treat. 2004;83:189.