The subcutaneous (SC) formulation of rituximab was approved by the US Food and Drug Administration (FDA) in June 2017.1 SC rituximab is currently FDA approved for the treatment of follicular lymphoma, diffuse large B-cell lymphoma (DLBCL), and chronic lymphocytic leukemia (CLL). In addition to these indications, the National Comprehensive Cancer Network (NCCN) guidelines recommend the use of SC rituximab for the treatment of other hematologic malignancies for which rituximab is included as part of the treatment regimen.1-3
Rituximab is a monoclonal antibody directed against the CD20 antigen on the surface of pre-B and mature B lymphocytes that results in cellular toxicity.1 SC rituximab is combined with hyaluronidase, which increases the absorption rate of rituximab by expanding the permeability of SC tissue through temporary depolymerization of hyaluronan.1
Studies have shown that the SC formulation of rituximab is as safe and effective as the intravenous (IV) formulation.4-6 Studies comparing SC and IV formulations of rituximab have shown that the SC formulation is noninferior to the IV formulation in its abilities to reach therapeutic area under the curve concentrations and to maintain appropriate serum trough concentrations.4-7
The dosing and administration duration of SC rituximab depend on the specific indication. For CLL, the approved dosing is a fixed dose of rituximab 1600 mg per 26,800 units of hyaluronidase, administered subcutaneously over 7 minutes.1 For DLBCL and for follicular lymphoma, the approved dosing is a fixed dose of rituximab 1400 mg per 23,400 units of hyaluronidase, administered subcutaneously over 5 minutes.1 For all indications, the patient must receive at least 1 full dose of IV rituximab without having a severe adverse event before initiating treatment with the SC formulation.1
Severe adverse events with the SC formulation of rituximab include pulmonary events, such as bronchospasm, in addition to fever, chills, rigors, hypotension, angioedema, and urticaria.1 Similar to the IV formulation, to help mitigate the risk for an infusion-related reaction with SC rituximab, an antipyretic and an antihistamine are recommended to be administered before each dose of the drug, and adding a glucocorticoid can be considered, when necessary.1
The SC formulation allows patients to receive rituximab in 5 to 7 minutes compared with approximately 90 minutes with the IV formulation. After the administration of SC rituximab, patients are required to be monitored for 15 minutes.1 In addition to saving chair and nursing time, SC rituximab saves compounding time, because the vials are ready to use, unlike IV rituximab, which has to be compounded by the pharmacy.8,9 SC rituximab also results in cost-savings, by reducing waste, because it is administered as a flat dose.
In February 2019, Beth Israel Deaconess Medical Center in Boston, MA, approved the addition of SC rituximab for use in the outpatient hematology/oncology clinic. Patients are eligible to receive the SC formulation after receiving at least 1 dose of the IV formulation and having no severe adverse reactions.
The goal of this study was to evaluate the use of SC rituximab and the safety of its administration, and to estimate the saved nursing time by using SC rituximab versus IV rituximab in a cancer center.
Methods
In this retrospective, single-center study, we identified patients who received SC rituximab between February 1, 2019, and September 15, 2019, at Beth Israel Deaconess Medical Center, Boston, MA. All patients aged ≥18 years who received at least 1 dose of SC rituximab during this period were included in the study. Multiple administrations of rituximab were allowed for the same patient. The SC rituximab doses were drawn from ready-to-use vials by the institution’s pharmacy technicians in the cleanroom of the infusion pharmacy.
Our financial clearance team uses Craneware’s InSight software to determine if clinic-administered medications require prior authorization, as determined by the patients’ medical insurance, or if the medication is approved (meaning it passed review for medical necessity). Documentation in the medical record is only completed for medications that require prior authorization.
The medical chart review included hematology/oncology clinic provider notes, nursing documentation of SC rituximab administration, and medication orders in the pharmacy system. The data we collected included patient demographics, diagnosis, chemotherapy regimen, SC rituximab dose, administration-related adverse reactions, other adverse events, supportive care medications, and administration time. This study was deemed exempt by the local Institutional Review Board.
The primary objective of the study was to evaluate the safety of administration of SC rituximab in the cancer center. The secondary objective included quantifying nursing time (administration plus monitoring period) to estimate the time-savings with the use of SC rituximab compared with the assumed IV rituximab infusion time.
Descriptive statistics were used to characterize the study population. Continuous variables were reported as means and medians, with variance and standard deviation. Categorical variables were reported as counts with frequencies. No statistical test was performed. All analyses were performed using Microsoft Excel 2016.
Results
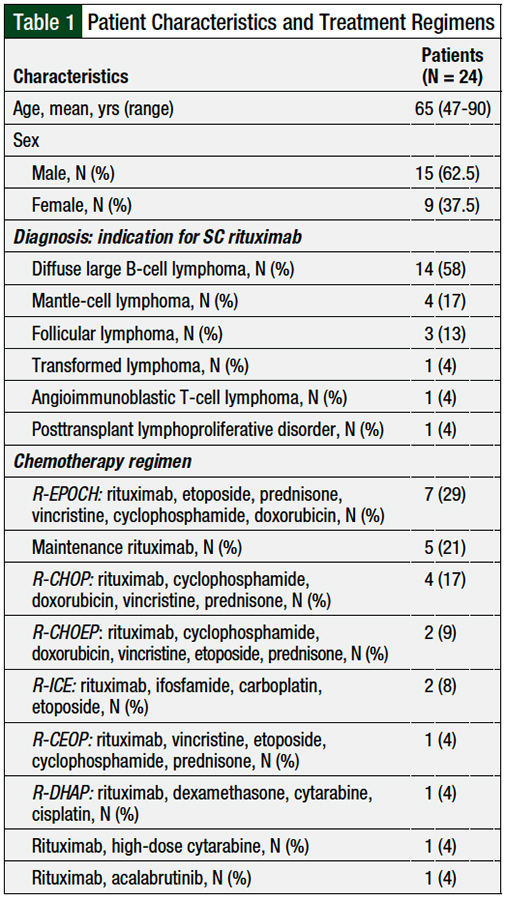
A total of 24 patients received SC rituximab at our institution during the study period, with a total of 61 SC administrations. All 24 patients received SC rituximab for the treatment of hematologic malignancies; the most common malignancy was DLBCL (58%; N = 14), followed by mantle-cell lymphoma (17%; N = 4) and follicular lymphoma (13%; N = 3). The patients’ characteristics are shown in Table 1.
The chemotherapy regimens administered to the patients are described in Table 1. The most common treatment regimens included rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (29%; N = 7), followed by maintenance rituximab (21%; N = 5) and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (17%; N = 4).
The financial clearance team confirmed the insurance approval for SC rituximab for 14 (58%) of the 24 patients in this study. No documentation on insurance approval or denial was available for the remaining 10 patients.
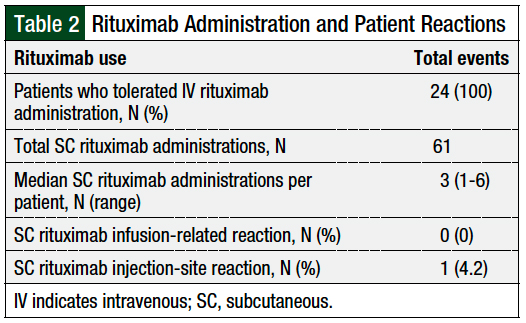
All 24 patients received and tolerated an IV rituximab dose before receiving the SC formulation. For premedications, all patients received acetaminophen and an antihistamine. The majority of the patients received diphenhydramine (83.33%) and the remaining patients received fexofenadine (16.66%). One patient also received famotidine, and 2 patients received montelukast in addition to fexofenadine or diphenhydramine. Two (8.33%) patients received prednisone before the administration of SC rituximab. None of the patients had infusion-related reactions with SC rituximab. However, 1 (4.2%) patient had an injection-site reaction, resulting in the discontinuation of SC rituximab (Table 2).
This patient had a diagnosis of mantle-cell lymphoma and received the rituximab plus high-dose cytarabine regimen (Table 1). He was admitted to the hospital because of febrile neutropenia, sore throat, and cough 5 days after the first dose of SC rituximab was administered. During that admission, a rash at the SC rituximab injection site was identified. The patient received empiric treatment for febrile neutropenia with cefepime, and vancomycin was added because of the rash at the SC rituximab injection site. No source of infection was identified, and the medical team suspected that the patient had a viral upper respiratory infection. The rash resolved during the patient’s 7-day hospitalization. No dermatology consultation was necessary. Thereafter, the patient received IV rituximab, with no further adverse sequelae.
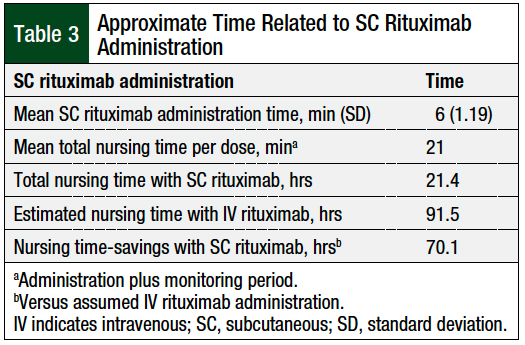
The mean administration time of SC rituximab was 6 minutes, with a standard deviation of 1.19 minutes (Table 3). The patients were monitored for 15 minutes after each dose of SC rituximab, as is indicated in the drug’s prescribing information1 and by our cancer center protocol. The mean total nursing time per dose, which included administration and a monitoring period, was 21 minutes. The total nursing time was 21.4 hours for the 61 doses included in this study (Table 3).
At our institution, an accelerated infusion protocol of IV rituximab over 90 minutes is available for patients who completed the standard initial IV infusion of rituximab, of approximately 300 minutes, without infusion-related reactions.
If the 61 doses administered in this study were given as an accelerated 90-minute IV infusion of rituximab, the assumed cumulative administration time would have been 91.5 hours. The use of SC rituximab resulted in an assumed mean time-savings of 69 minutes per dose, and an estimated total time-savings of 70.1 hours compared with the accelerated infusion of IV rituximab (Table 3).
Discussion
Every patient in this study appropriately received and tolerated an IV infusion of rituximab before the administration of SC rituximab. SC rituximab was only administered for hematologic malignancy indications, as approved by the FDA and/or as recommended by the NCCN guidelines.1-3 The supportive care premedications were similar and consistent for all patients.
All patients tolerated the administration of SC rituximab, and no infusion-related reactions were reported. One (4.2%) patient reported an injection-site reaction that resulted in the discontinuation of SC rituximab.
In a study comparing the IV and SC formulations of rituximab in patients with follicular lymphoma, 79% of patients in each group reported 1 or more adverse events.7 The patients who received SC rituximab had a higher incidence of administration-related reactions than the patients who received IV rituximab (31% vs 4%, respectively), with erythema being the most common reaction (13%).7
In a study comparing IV and SC formulations of rituximab in patients with DLBCL, administration-related reactions were reported in 20.9% of patients who received SC rituximab and in 21.3% of patients who received IV rituximab.6 Injection-site reactions occurred in 5.7% of patients who received SC rituximab and in none of the patients who received IV rituximab.6
Our findings show a lower frequency of injection-site reactions compared with these clinical trials. This could be a result of the small sample size of our retrospective study. Furthermore, decreased reporting of injection-site reactions by patients should be considered, because the reaction could have resolved by the next time they were seen in the hematology/oncology clinic.
The total nursing time for SC rituximab, which includes the administration and monitoring period, was 21.4 hours for the 61 doses in this study. The assumed cumulative administration time with IV rituximab for the same 61 doses would have been 91.5 hours. By using the SC rituximab formulation, nursing time was reduced by 76.6%, with an estimated total time-savings of 70.1 hours.
A time-savings study by De Cock and colleagues includes data from 8 countries and shows that the average injection time for SC rituximab was 8.3 minutes (95% confidence interval, 7.0-9.6).9 This was similar to our finding of a mean administration time of 6 minutes, ranging from 5 to 10 minutes.
The study by De Cock and colleagues also followed a patient’s chair time using a stopwatch to measure accurately the time spent in clinic to compare the IV and SC administration time of rituximab.9 The mean chair time was 262.1 minutes for IV rituximab compared with a mean chair time of 67.3 minutes for SC rituximab.9
We did not use a stopwatch to measure the total time a patient spent in the clinic, because our study was a retrospective chart review. The entire duration of time that patients spent in the clinic for the administration of rituximab is unavailable. We collected the information on rituximab administration time from nursing documentation. The estimated time-savings in our study shows the potential to decrease patient time in clinic, increase appointments available in the infusion clinic, and decrease the nursing workload.
Future directions at our institution include extending the study period to increase the number of patients and the SC rituximab doses, and ensuring that injection-site reactions or adverse events are identified. In addition, chair time and cost-saving analysis should be considered in future studies.
Limitations
This study has several limitations, including the known limitations related to a retrospective chart review. The documentation of injection-site reactions or other adverse events relied on patients reporting them to the clinic after the SC rituximab administration. In addition, we relied heavily on nursing and provider documentation for data collection. To ensure that all available data were captured, all inpatient and outpatient hematology/oncology notes were reviewed, starting on the day that SC rituximab was administered for the first time for each patient.
A major limitation is the small sample size in a single institution.
Furthermore, a lack of documentation in our institution of insurance approval made it impossible to guarantee that all SC rituximab doses were approved by the patient’s insurance. The current practice in our center includes documentation only when a prior authorization is required by the health plan. No documentation is completed when the medication passes review by medical necessity. The financial clearance team is not under pharmacy leadership.
Finally, time-savings cannot be confirmed in a retrospective review, because the patients received at least 1 dose (ie, the first dose) of IV rituximab infusion and then were switched to SC rituximab, per the prescribing information. We assumed that the nurses would have administered the next doses of IV rituximab over 90 minutes (per protocol) if SC rituximab was not available.
Conclusion
Our findings showed that use of the SC rituximab formulation in our cancer center was well-tolerated. SC rituximab was used for the appropriate indication after at least 1 administration of IV rituximab, and was dosed appropriately. SC rituximab resulted in estimated time-savings by decreasing the nursing time spent and decreasing the nursing workload. This saved time can be used for the care of other patients and in the administration of other treatments, improving the efficiency of the hematology/oncology infusion clinic, and decreasing the time patients spend in the clinic. The infusion chair time saved, and a cost analysis, should be explored in prospective studies.
Author Disclosure Statement
Dr Patel, Mr Patel, and Dr Mejías-De Jesús have no conflicts of interest to report.
References
- Rituxan Hycela (rituximab and hyaluronidase human) injection, for subcutaneous use [prescribing information]. Genentech; June 2021. www.gene.com/download/pdf/rituxan_hycela_prescribing.pdf. Accessed November 29, 2021.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Version 1.2022. September 8, 2021. www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Accessed November 29, 2021.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): B-Cell Lymphomas. Version 5.2021. www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed November 29, 2021.
- Assouline S, Buccheri V, Delmer A, et al. Pharmacokinetics, safety, and efficacy of subcutaneous versus intravenous rituximab plus chemotherapy as treatment for chronic lymphocytic leukaemia (SAWYER): a phase 1b, open-label, randomised controlled non-inferiority trial. Lancet Haematol. 2016;3:e128-e138.
- Davies A, Merli F, Mihaljevic B, et al. Pharmacokinetics and safety of subcutaneous rituximab in follicular lymphoma (SABRINA): stage 1 analysis of a randomised phase 3 study. Lancet Oncol. 2014;15:343-352.
- Lugtenburg P, Avivi I, Berenschot H, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017;102:1913-1922.
- Salar A, Avivi I, Bittner B, et al. Comparison of subcutaneous versus intravenous administration of rituximab as maintenance treatment for follicular lymphoma: results from a two-stage, phase IB study. J Clin Oncol. 2014;32:1782-1791.
- Carlson J, Cox K, Bedwell K, Ku M. Rituximab for subcutaneous delivery: clinical management principles from a nursing perspective. Int J Nurs Pract. 2015;21(suppl 3):1-13.
- De Cock E, Kritikou P, Sandoval M, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One. 2016;11:e0157957. doi: 10.1371/journal.pone.0157957.