Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in adults (aged ≥20 years) in the United States, accounting for 38% of all leukemias.1 In 2019, an estimated 200,766 Americans had CLL, and an estimated 20,160 new cases of CLL and 4410 deaths from CLL were estimated to occur in 2022 in the United States.2
CLL is characterized by the progressive accumulation of small, mature B-lymphocytes within the blood, bone marrow, and lymphatic system.3,4 Small lymphocytic lymphoma (SLL) is the same malignancy as CLL but is located in the lymph nodes and spleen and not in the blood.5
CLL/SLL is an indolent disease, with a 5-year relative survival rate of 87.9%.2 The management of asymptomatic CLL/SLL often starts with surveillance and transitions to intervention with disease progression or another indication for treatment.6,7
Novel agents have been available for the treatment of CLL/SLL since the 2014 US Food and Drug Administration (FDA) approval of ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor.8 Since then, additional novel agents have received FDA approval for CLL/SLL, including a BCL2 inhibitor (venetoclax), PI3K inhibitors (duvelisib, idelalisib), and a second-generation BTK inhibitor (acalabrutinib).8
Although the per-patient lifetime cost of CLL treatment is estimated to be 4-fold higher with a novel agent than with chemoimmunotherapy ($604,000 vs $147,000, respectively),9 the favorable risk-benefit profiles of novel agents have led to a shift away from cytotoxic therapies and to a rapid adoption of the novel agents as the standard of care for patients with CLL/SLL.10-15
Despite the improved efficacy, tolerability, and safety of the novel agents compared with chemoimmunotherapy, they can be associated with adverse events (AEs). The safety profiles of the novel agents differ.16-25
Several cardiovascular (CV) events have been associated with the novel agents, specifically with the BTK inhibitors. Ibrutinib has been linked to supraventricular arrhythmias (especially atrial fibrillation), ventricular arrhythmias, hypertension, heart failure, conduction disorders, central nervous system ischemic events, and hemorrhagic events.26,27 The second-generation BTK inhibitor, acalabrutinib, has demonstrated noninferior progression-free survival, with fewer CV AEs compared with ibrutinib.28
CLL/SLL is primarily seen in older patients, who often have preexisting comorbidities, and an increased risk for CV events.29,30 The 2018 annual mean cost of CV events per patient in the general US population was substantial, and was estimated to be $61,864 for myocardial infarctions, $49,427 for heart failure, $46,162 for stroke, $31,860 for unstable angina, and $25,306 for transient ischemic attacks.31
The impact of CV events on healthcare utilization and costs for patients with CLL/SLL who are receiving a novel agent is unknown. The goal of this analysis was to evaluate the prevalence of CV events in patients with CLL/SLL in the first 12 months of a novel agent initiation and the real-world per-patient healthcare costs associated with the CV events.
Methods
In this retrospective, observational study, we used administrative US healthcare claims data to evaluate CV events and healthcare resource utilization and costs incurred by patients with CLL/SLL who received a novel agent. The population of patients with CLL/SLL was derived from the IBM MarketScan Commercial Claims and Encounters (ie, Commercial) and MarketScan Medicare Supplemental (ie, Medicare) databases.
The Commercial database contains the integrated patient-level medical (inpatient and outpatient) and outpatient pharmacy data of employees and their dependents, who are covered under a variety of fee-for-service and managed care health plans. The Medicare database contains the healthcare data (medical and pharmacy) of retirees with Medicare supplemental insurance paid by employers. The Medicare-covered portion of payment and the employer-paid portion are included in this database.
All Commercial and Medicare database records are statistically de-identified and certified to be fully compliant with US patient confidentiality requirements set forth in HIPAA (Health Insurance Portability and Accountability Act of 1996). Because we included only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
To be eligible to participate in this study, patients had to be aged ≥18 years and have at least 1 pharmacy claim for a novel agent (ie, acalabrutinib, duvelisib, ibrutinib, idelalisib, or venetoclax) for CLL or SLL between November 1, 2013, and November 30, 2019. The date of the first pharmacy claim for a novel agent was designated as the index date.
Patients had to have at least 1 inpatient or 2 outpatient “nonruleout claims” with a diagnosis of CLL or SLL at least 14 days apart in the 6 months before the index date (ie, the baseline period). Nonruleout claims are claims for a professional encounter (not for a laboratory test or an imaging study) that was used to diagnose a condition.
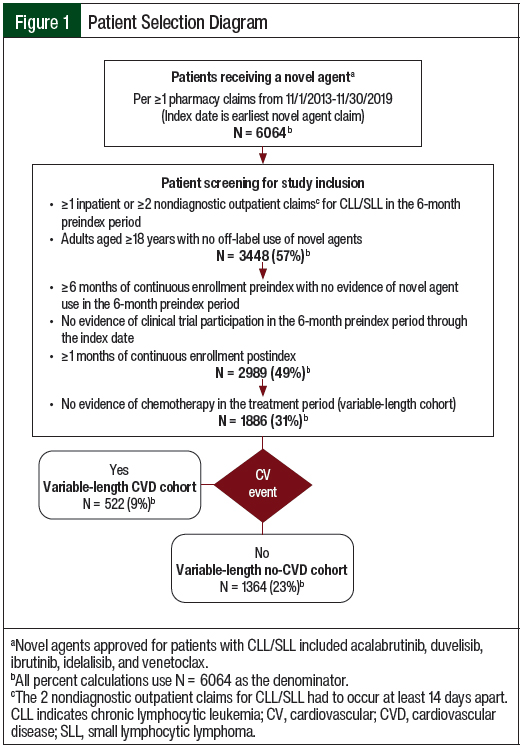
The use of 2 diagnostic codes within a defined time frame has a high positive predictive value for lymphoma.32 Patients were also required to have continuous enrollment in an insurance plan with medical and pharmacy benefits for the 6-month baseline period through a minimum of 1 month after the index date (Figure 1).
Patients were excluded if they were diagnosed with CLL/SLL and with mantle-cell lymphoma, received one of the CLL/SLL novel agents, or had a diagnostic code that was indicative of clinical trial participation in the 6-month baseline period. Patients with a claim for chemotherapy given not for CLL during the treatment period were excluded as a proxy for patients receiving active treatment for other types of cancer. However, patients who received chemotherapy after treatment with a novel agent for CLL/SLL ended were not excluded from this analysis.
The patients were grouped into 2 mutually exclusive cohorts based on claims for a CV disease (CVD) diagnosis during the novel agent treatment period: patients with evidence of a CV event were in the CVD cohort, and patients without evidence of a CV event were in the no-CVD cohort. The codes used to identify CV events are provided in the Appendix Table 1.
The patient characteristics were captured in the fixed 6-month baseline period; the outcomes were evaluated in a variable-length follow-up period (ie, minimum 1 month; maximum 12 months). The novel agent treatment period was defined as the day from the initiation of a novel agent treatment through the earliest of the last novel agent claim plus 60 days, the end of continuous enrollment, or the end of the study period. The discontinuation of a novel agent was defined as a gap of ≥60 days between prescription claims. The study time periods are shown in the Appendix Figure 1.
Outcome Measures
CV events were identified in the 6-month baseline period and in the novel agent treatment period up to 1 year after its initiation, based on at least 1 inpatient or outpatient nonruleout claim with a diagnosis for a CV event (Appendix Table 1); the results of using this method have been previously published.33
The CV events captured in this analysis were based on CV events associated with ibrutinib in a previously published study and included cardiomyopathy, conduction disorders, heart failure, new-onset hypertension, myocardial infarction, supraventricular arrhythmias, and ventricular arrhythmias.26
The lack of clinical information in claims data prevents the possibility of identifying any changes in existing hypertension. Therefore, only patients with new-onset hypertension (ie, no hypertension claim at baseline) were flagged in the treatment period. Patients with any other CV events were included, regardless of evidence of having such CV events at baseline.
The first claim with a diagnosis for any CV events in the variable-length follow-up period defined the CV event date. Patients with claims for multiple CV events were included in all the CV categories for which they had a CV claim. Patients with multiple claims for the same CVD category were only represented in the first instance; all subsequent claims were considered to be related to the first instance of a CV event.
All-cause healthcare resource utilization and costs included all medical and pharmacy claims, regardless of the diagnosis, procedure, or drug codes on the claim. All-cause healthcare resource utilization and cost were evaluated in the variable-length follow-up period and were calculated for the pre– and post–CV event periods. Healthcare resource utilization and cost reported in the variable-length follow-up period were annualized to estimate the annual costs, and those that were evaluated in the pre– and post–CV event periods were presented as per-patient per-month (PPPM) costs to standardize outcomes across the variable-length periods.
Healthcare services included hospital admissions, outpatient services (ie, emergency department, physician office visits, and other outpatient services), and outpatient pharmacy prescriptions. “Other” outpatient services included claims for imaging procedures and laboratory tests. Total healthcare costs included the expenditures incurred to the healthcare system from medical and pharmacy services. Costs were computed as the paid amounts of adjudicated claims, including insurer payments and patient cost-sharing components. All costs were presented in 2019 US dollars and were adjusted per the Medical Care component of the Consumer Price Index.34
To elucidate changes in healthcare costs regarding a CV event date, costs were assessed in 30-day periods. The CV event month (ie, period 0) was defined as 15 days before the CV event date through 14 days after the event date. The 30-day periods were defined from the CV event month up to 12 consecutive periods before and after a CV event. Only patients with continuous enrollment for the entire 30 days were included in that period, so the sample size differed across periods.
Patients’ demographics were measured on the date of initiating a novel agent and included age, sex, insurance plan type, primary payer, geographic region, urbanicity, and index year. Clinical characteristics were captured during the 6-month baseline period and included the National Cancer Institute (NCI)-adapted Deyo-Charlson Comorbidity Index (CCI); CV comorbidities (eg, atrial fibrillation, deep-vein thrombosis, hyperlipidemia, major bleeding, and pulmonary embolism); concomitant medications (eg, antiarrhythmic drugs, anticoagulants, antidepressants, antihypertensive drugs, antiplatelet drugs, antipsychotic drugs, hormonal birth control, and phosphodiesterase-5 inhibitors); evidence of another primary cancer in the baseline period via a diagnosis for a primary cancer other than CLL or SLL, or a claim for any chemotherapy agent; and baseline all-cause total healthcare costs.
Comorbid conditions were identified via International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification diagnosis codes. Medications were defined by the National Drug Codes and Healthcare Common Procedure Coding System codes.
Statistical Analyses
Study measures were summarized as count and percentage for categorical variables, and as median and interquartile range (IQR) and/or mean and standard deviation (SD) for continuous variables. Statistical comparisons between the CVD and no-CVD cohorts were evaluated using chi-square tests for the categorical variables and Student’s t-tests for the continuous variables. The unadjusted utilization and cost measures were calculated as PPPM before and after the CV events, and were annualized for the variable-length follow-up period.
Multivariable generalized linear models were used to evaluate the impact of a CV event on the annualized all-cause total healthcare costs of patients with CLL/SLL in the variable-length follow-up period. The model was adjusted for relevant baseline demographics, clinical comorbidities, and healthcare costs before the initiation of a novel agent. Because of the modest sample size of patients with CLL/SLL in this analysis, the number of model covariates was limited to avoid overfitting. A P value of <.05 was considered to be statistically significant.
Results
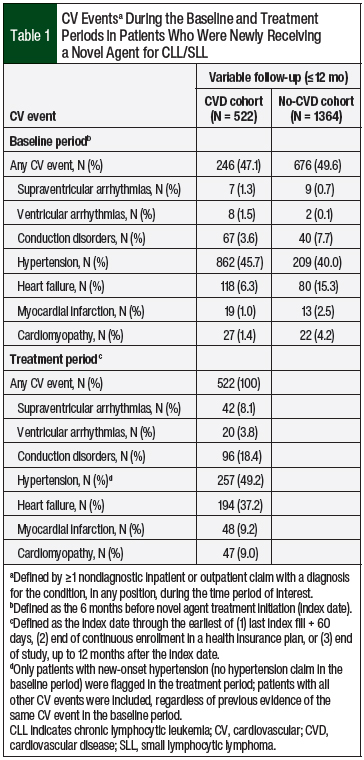
Of the 6064 patients with a pharmacy claim for a novel agent during the study period, 3448 (56.9%) had a diagnosis of CLL/SLL. After applying the inclusion and exclusion criteria, 1886 patients were eligible for the study (Figure 1). At the median variable-length follow-up of 305 days (IQR, 184-365 days), 522 (27.7%) of the patients had a claim for a CV event in the treatment period (Table 1).
The patients in the CVD cohort had their first CV event at a median of 74.5 days (IQR, 22-158 days) and a mean of 103 days (SD, 93.9 days) after initiating a novel agent (ie, pre–CV event period). The post–CV event period was longer than the pre–CV event period, with a median length of 178 days and a mean of 180.7 days (SD, 133.1 days). New-onset hypertension was the most common (49.2%) CV event, followed by heart failure (37.2%) and conduction disorders (18.4%; Table 1).
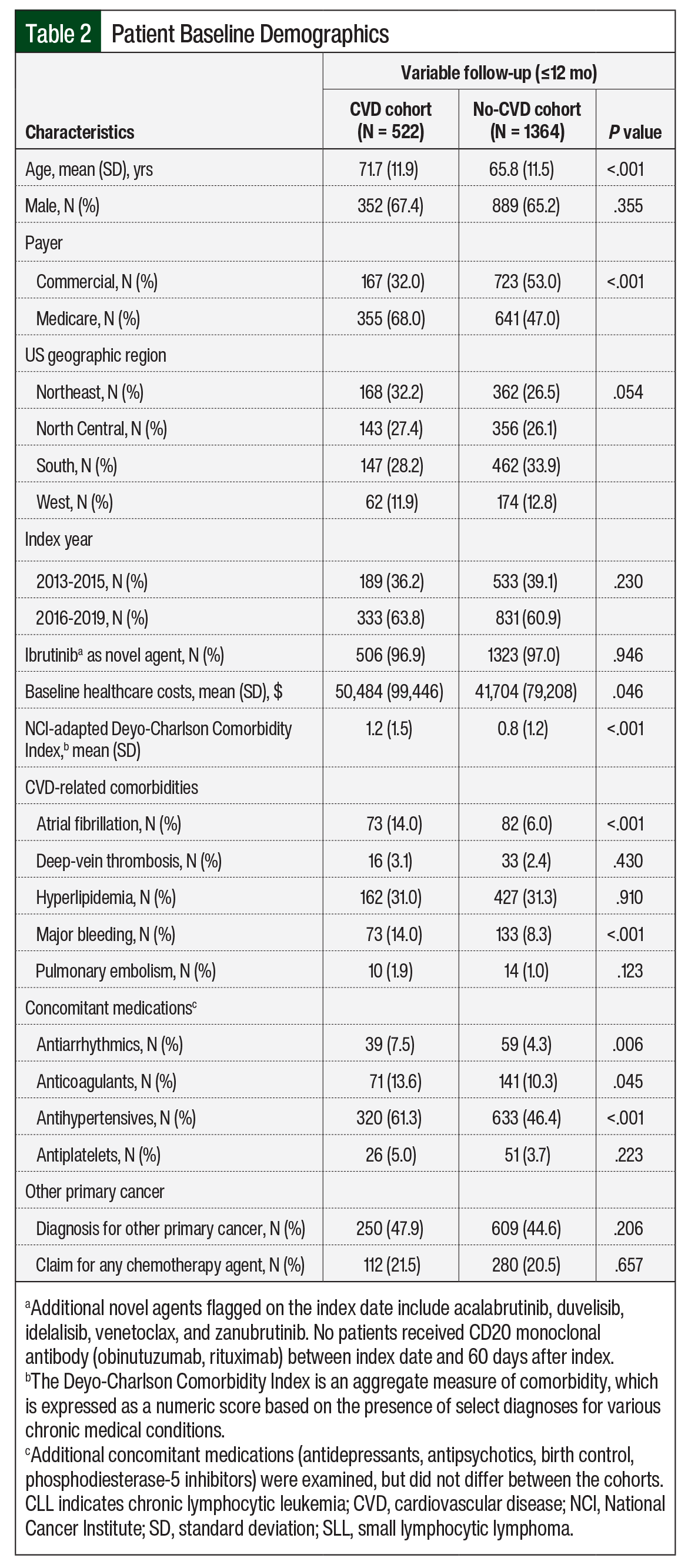
Patients in the CVD cohort were older than in the no-CVD cohort (mean age, 71.7 years vs 65.8 years, respectively; P <.001), and more patients in that cohort had Medicare coverage (68% vs 47%, respectively; P <.001). Patients’ baseline clinical characteristics are shown in Table 2.
Disease burden, assessed via the NCI-adapted CCI, was greater in the CVD cohort than in the no-CVD cohort (mean, 1.2 vs 0.8, respectively; P <.001). Baseline use of antihypertensives (61.3% vs 46.4%, respectively; P <.001), antiarrhythmic drugs (7.5% vs 4.3%, respectively; P = .006), and anticoagulants (13.6% vs 10.3%, respectively; P = .045) was higher in the CVD cohort than in the no-CVD cohort. In both cohorts, >65% of patients were male, and 97% of the patients received ibrutinib as the index drug. More than 44% of the patients had cancer other than CLL/SLL, and >20% received chemotherapy at baseline (Table 2).
The annualized healthcare resource utilization and cost were greater in the CVD cohort than in the no-CVD cohort for hospital admissions (50.4% vs 22.1%, respectively) and emergency department visits (48.7% vs 28.4%, respectively; all P <.001; Appendix Table 2).
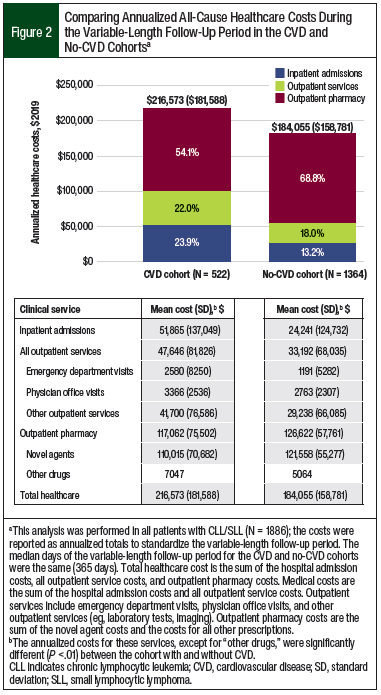
Patients in the CVD cohort had more hospital admissions (1.35 vs 0.55, respectively), emergency department visits (1.59 vs 0.79, respectively), outpatient physician office visits (24.80 vs 20.93, respectively), other outpatient services (62.46 vs 39.50, respectively), and outpatient pharmacy prescriptions (50.94 vs 43.02, respectively) than the no-CVD cohort (all P <.001). This greater healthcare utilization for the CVD cohort versus the no-CVD cohort corresponded to higher unadjusted annualized medical costs, with increased spending on hospital admissions ($51,865 vs $24,241, respectively; P <.001) and outpatient services ($47,646 vs $33,192, respectively; P <.001; Figure 2).
The annualized novel agent costs contributed greatly to the annualized total outpatient pharmacy costs in both cohorts. In the CVD cohort, 94% ($110,015 of $117,062) of the total outpatient pharmacy cost was novel agent expenses; in the no-CVD cohort that percentage was 96% ($121,558 of $126,622; Figure 2). Despite higher annualized outpatient pharmacy prescriptions in the CVD cohort than in the no-CVD cohort (51 vs 43, respectively; P <.001), the outpatient pharmacy costs (P = .003) and novel agent costs (P <.001) were higher in the no-CVD cohort.
The annualized non–novel agent pharmacy costs were 1.4-fold higher in the CVD cohort than in the no-CVD cohort ($7047 vs $5064, respectively), which indicates the prevalent use of non–novel agent medications after a CV event. The high medical costs contributed to a greater unadjusted, annualized, all-cause total healthcare cost in the CVD cohort compared with the no-CVD cohort ($216,573 vs $184,055, respectively; P <.001; Figure 2).
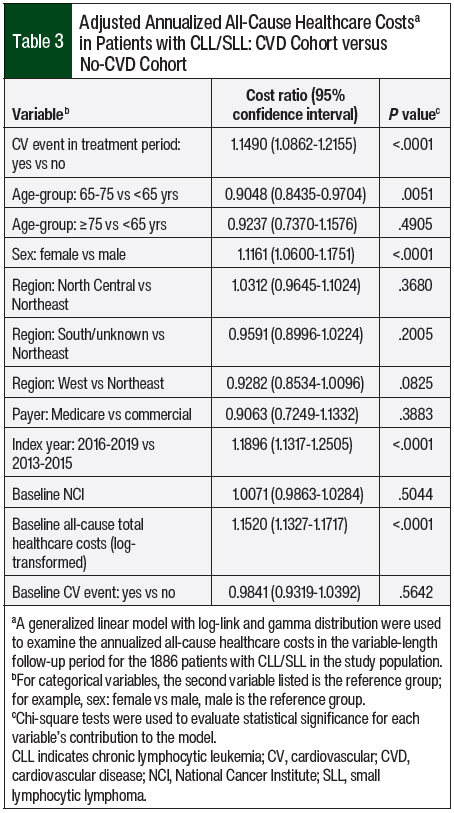
Adjusting for patients’ baseline characteristics, the annualized all-cause costs in the CVD cohort were 1.15 times higher than in the no-CVD cohort (P <.001). Patients’ age, sex, index year, and baseline all-cause healthcare costs were significant drivers of annualized all-cause healthcare costs (Table 3).
As expected, healthcare utilization was lower before than after a CV event, including the PPPM counts of hospital admissions (0.05 vs 0.16, respectively), emergency department visits (0.09 vs 0.14, respectively), outpatient physician office visits (1.89 vs 1.93, respectively), and other outpatient services (3.74 vs 5.37, respectively). The associated PPPM medical costs were also lower before than after a CV event, including less spending on hospital admissions ($1259 vs $7190, respectively) and outpatient services ($2934 vs $4155, respectively; Figure 3). The main drivers of total healthcare costs differed in the pre– and post–CV event periods, with PPPM cost contributions from pharmacy (75% vs 39%, respectively), outpatient services (18% vs 22%, respectively), and hospitalization (8% vs 39%, respectively).
However, the PPPM outpatient pharmacy costs were higher before than after CV event ($12,425 vs $7186, respectively), which was driven by higher PPPM costs of novel agents ($11,932 vs $6667, respectively) used before a CV event. Despite the higher PPPM pharmacy costs before a CV event, the lower PPPM medical costs led to lower total PPPM healthcare costs before a CV event than after it ($16,619 vs $18,531, respectively).
The total healthcare cost was highest in the CV event month ($32,544) and decreased to pre–CV event levels in the 12 months after the event. The spike in total healthcare cost was driven by an increased hospital admission cost ($17,461) and outpatient services cost ($6492) in the CV event month. Despite decreased medical costs in the 30-day post–CV event period, inpatient and outpatient services costs remained higher after the CV event than in the 30 days before the event.
Consistent with the trends observed for all-cause healthcare costs in the pre– and post–CV event periods, novel agent costs were highest 9 months before a CV event ($11,040), and decreased through the CV event month to 9 months later ($5700; Appendix Figure 2).
Discussion
Overall, 27.7% of the patients with CLL/SLL had a claim for a CV event in the first year of using a novel agent. The median time to the first CV event was 74.5 days after initiating therapy. In the first year of a novel agent use, patients in the CVD cohort had higher all-cause healthcare costs than those in the no-CVD cohort.
As expected, all-cause healthcare costs were higher after a CV event than before it. The outpatient pharmacy costs decreased after the CV event as a result of a decrease in the novel agent costs. However, the termination or interruption of treatment with a novel agent may result in nonoptimal clinical outcomes, including a risk for early CLL/SLL progression.35,36
The management of CLL/SLL after a CV event is not well-studied. A review of clinical trials showed that many patients continued to use ibrutinib after atrial fibrillation, and >50% received anticoagulant and antiplatelet therapy.37 Clinical trial outcomes, however, may not be representative of real-world settings. The increase in pharmacy costs not related to novel agents may be directly or indirectly related to the care of patients with CLL/SLL, but additional analyses are needed.
Although CV events associated with novel agents in patients with CLL/SLL have been well-documented,26,27 the costs associated with CV events have not been previously reported. A MarketScan analysis that examined all-cause healthcare costs of patients with CLL/SLL stratified by the number of AEs showed a 6.7-fold increase in the mean monthly cost of patients with ≥6 AEs ($6032) compared with the cost for patients with no AEs ($905).38 However, that study included common chemotherapy-related AEs (ie, neutropenia, dehydration, fatigue, nausea) in addition to CV events (ie, atrial fibrillation, hypertension, hemorrhage), and the economic burden of individual CV events was not evaluated.38
In another study, patients with CLL and atrial fibrillation had twice the odds of hospitalization and 44% higher total costs versus patients without atrial fibrillation; that study, however, did not account for treatment with novel agents.39 Our real-world analysis supports these findings, showing 1.2-fold greater adjusted annual healthcare costs in the CVD cohort than in the no-CVD cohort. Other studies have shown that CV events can lead to significant downstream costs,40,41 but our current study was limited to 1 year of follow-up, which may underrepresent the downstream costs of CV events.
The total healthcare cost of patients with CLL/SLL in our study was comparable to the all-cause total healthcare costs of $95,316 to $174,240 reported in previous studies of patients with CLL/SLL who were receiving novel agents.36,38,42 In the MarketScan analysis that evaluated patients diagnosed with CLL/SLL between 2012 and 2015, patients who received systemic therapy had a mean monthly all-cause healthcare cost of $7943, which rose to $21,766 in the months of ibrutinib monotherapy.38
A 2013-2015 Optum analysis of US adults with CLL reported a mean total healthcare cost of $97,477 (SD, $37,940) in patients who received at least 9 months of first-line ibrutinib monotherapy.42
Although novel agents have demonstrated benefits in CLL/SLL versus traditional chemoimmunotherapy, differences have been noted in the safety profiles of the various novel agents.8,10-15,43 Ibrutinib has been associated with an increased risk for CV AEs, mainly atrial fibrillation and hypertension.26,27,44 By contrast, in the ELEVATE-RR study, the rates of CVD-related AEs were lower in patients with CLL who received acalabrutinib versus ibrutinib, while maintaining comparable efficacy.28
A recent systematic review also reported a lower incidence of AEs, including atrial fibrillation and hypertension, among patients with B-cell lymphoproliferative disorders who received acalabrutinib compared with those who received ibrutinib.18 Acalabrutinib has been uniquely associated with an increased risk for headaches.15,19
Fewer CVD-related AEs have been documented with the investigational second-generation BTK inhibitor zanubrutinib in a single-arm phase 2 study, in which approximately 85% of patients with CLL/SLL had a partial or a complete response to therapy and a 12-month AE-free rate of 92.9%.20
Venetoclax has been associated with few CV AEs45; however, neutropenia and tumor lysis syndrome have been reported.46,47
Despite demonstrating efficacy in patients with CLL/SLL, the use of the PI3K inhibitors versus chemoimmunotherapy has been limited, because of immune-mediated AEs and infection complications.21,22
Limitations
This study had several limitations, including its retrospective and observational design.
Because of our study period, our data mainly reflect ibrutinib therapy; thus, comparisons between the other CLL novel agents (ie, acalabrutinib, duvelisib, idelalisib, or venetoclax) were not possible.
In addition, we focused on CV events occurring within the first 12 months of initiating a novel agent, which may underrepresent the total costs and AEs that can occur after the initial year of treatment.
Furthermore, as in all administrative claims analyses, this study may have coding limitations and data entry errors.
Moreover, the generalizability of the study findings is limited to patients with commercial or Medicare insurance, and may not be representative of patients with other types of health insurance, those without insurance, or patients outside of the United States.
Finally, most patients with CLL live for many years, and the duration of novel agent use may be years; this limited the ability of our 12-month analysis to estimate the total healthcare costs of the lengthy natural history of CLL.
Conclusions
The current study updates previous examinations of healthcare costs among patients with CLL/SLL. To our knowledge, this is the first study to compare healthcare costs in patients with CLL/SLL stratified by CV event status after the initiation of a novel agent. This study also adds to the literature information about the healthcare costs for patients with CLL/SLL, by analyzing costs in multiple times during the pre– and post–CV event periods, reporting the costs in 30-day periods before and after a CV event, and evaluating the adjusted annual healthcare costs by CV event status.
This real-world study shows that patients with CLL/SLL who have a CV event incur higher total all-cause healthcare costs in the first year after the initiation of a novel agent than patients without a CV event. These higher annual total healthcare costs are driven by increased medical costs, despite the decreased novel agent costs after the CV event, suggesting increased medical management and novel agent discontinuation or interruptions.
The clinical implications of these findings highlight the importance of minimizing CV events to reduce the total healthcare costs and avoiding early discontinuation of highly effective treatment for patients with CLL/SLL.
Future analyses should examine the total healthcare expenditures past the first year of using a novel agent and compare the CV events and costs for a longer period after additional novel agents are approved by the FDA for CLL/SLL. Research should also evaluate the clinical outcomes associated with CLL/SLL treatment disruptions caused by CV events.
Acknowledgments
Programming was provided by Helen Varker and Diana Stetsovsky of IBM Watson Health; statistical analyses were provided by David Smith of IBM Watson Health. These services were paid for by AstraZeneca. The study data came from IBM Watson Health; restrictions apply to these data.
Funding Source
This study was funded by AstraZeneca.
Author Disclosure Statement
Dr Malangone-Monaco, Dr Marchlewicz, Dr Lo, and Dr Huntington received funding from AstraZeneca for this study; Ms Ryan is an employee and shareholder of AstraZeneca; Dr Lo was previously an employee of AstraZeneca; Dr Huntington has provided consulting services to Janssen, AbbVie, Novartis, BeiGene, Flatiron Health, Genentech, Bayer, SeaGen, ADC Therapeutics, Tyme Inc, Merck, Epizyme, and TG Therapeutics.
References
- American Cancer Society. Cancer Facts & Figures 2022. 2022. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf. Accessed December 5, 2022.
- National Cancer Institute SEER program. Cancer stat facts: leukemia—chronic lymphocytic leukemia (CLL). https://seer.cancer.gov/statfacts/html/clyl.html. Accessed April 1, 2022.
- Zenz T, Mertens D, Küppers R, et al. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10:37-50.
- American Cancer Society. What is chronic lymphocytic leukemia? www.cancer.org/cancer/chronic-lymphocytic-leukemia/about/what-is-cll.html. Accessed December 5, 2022.
- American Cancer Society. Types of B-cell lymphoma. Updated January 29, 2019. www.cancer.org/cancer/non-hodgkin-lymphoma/about/b-cell-lymphoma.html. Accessed December 5, 2022.
- Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96:1679-1705.
- Parikh SA, Rabe KG, Kay NE, et al. Chronic lymphocytic leukemia in young (≤ 55 years) patients: a comprehensive analysis of prognostic factors and outcomes. Haematologica. 2014;99:140-147.
- Patel K, Pagel JM. Current and future treatment strategies in chronic lymphocytic leukemia. J Hematol Oncol. 2021;14:69. doi: 10.1186/s13045-021-01054-w.
- Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017;35:166-174.
- Byrd JC, Brown JR, O’Brien S, et al; for the RESONATE investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213-223.
- Seymour EK. Rapid transitions in the standard of care for chronic lymphocytic leukemia (CLL). Oncotarget. 2019;10:2484-2485.
- Burger JA, Tedeschi A, Barr PM, et al; for the RESONATE-2 investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425-2437.
- O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17:1409-1418.
- Olszewski AJ, Davids MS, Yakirevich I, Egan PC. Early adoption and outcomes of ibrutinib as treatment for older patients with chronic lymphocytic leukemia (CLL): a population-based study. Blood. 2019;134(suppl 1):265.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278-1291. Erratum in: Lancet. 2020;395:1694.
- Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3:1553-1562.
- Davids MS, Telford C, Abhyankar S, et al. Matching-adjusted indirect comparisons of safety and efficacy of acalabrutinib versus other targeted therapies in patients with treatment-naïve chronic lymphocytic leukemia. Leuk Lymphoma. 2021;62:2342-2351.
- Hilal T, Hillegass WB, Gonzalez-Velez M, et al. Adverse events in clinical trials of ibrutinib and acalabrutinib for B-cell lymphoproliferative disorders: a systematic review and network meta-analysis. Blood. 2020;136(suppl 1):23.
- Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849-2861.
- Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13:48. doi: 10.1186/s13045-020-00884-4.
- Zydelig (idelalisib) tablets, for oral use [prescribing information]. Gilead Sciences; February 2022. www.gilead.com/~/media/Files/pdfs/medicines/oncology/zydelig/zydelig_pi.pdf. Accessed December 6, 2022.
- Copiktra (duvelisib), capsules for oral use [prescribing information]. Secura Bio; December 2021. https://copiktrahcp.com/pdf/COPIKTRA-PI-USCPR2007403.pdf. Accessed December 6, 2022.
- Calquence (acalabrutinib) tablets, for oral use [prescribing information]. AstraZeneca; August 2022. https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/e2a005a7-65a0-4388-a671-dc887815a938/e2a005a7-65a0-4388-a671-dc887815a938_viewable_rendition__v.pdf. Accessed December 6, 2022.
- Venclexta (venetoclax tablets), for oral use [prescribing information]. AbbVie, Genentech; June 2022. www.rxabbvie.com/pdf/venclexta.pdf. Accessed December 6, 2022.
- Imbruvica (ibrutinib) capsules/tablets/oral suspension, for oral use [prescribing information]. Pharmacyclics, Janssen Biotech; August 2022. www.imbruvica.com/files/prescribing-information.pdf. Accessed December 6, 2022.
- Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74:1667-1678.
- Estupiñán HY, Berglöf A, Zain R, Smith CIE. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol. 2021;9:630942. doi: 10.3389/fcell.2021.630942.
- Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39:3441-3452.
- Shanafelt TD, Parikh SA, Noseworthy PA, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 2017;58:1630-1639.
- Thurmes P, Call TG, Slager SL, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49-56.
- Ryder S, Fox K, Rane P, et al. A systematic review of direct cardiovascular event costs: an international perspective. Pharmacoeconomics. 2019;37:895-919.
- Herman RA, Gilchrist B, Link BK, Carnahan R. A systematic review of validated methods for identifying lymphoma using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):203-212.
- Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7:e008740. doi: 10.1161/JAHA.118.008740.
- United States Bureau of Labor Statistics. Consumer Price Index. Revised October 2020. www.bls.gov/cpi/. Accessed December 6, 2022.
- Fürstenau M, Hallek M, Eichhorst B. Sequential and combination treatments with novel agents in chronic lymphocytic leukemia. Haematologica. 2019;104:2144-2154.
- Emond B, Sundaram M, Romdhani H, et al. Comparison of time to next treatment, health care resource utilization, and costs in patients with chronic lymphocytic leukemia initiated on front-line ibrutinib or chemoimmunotherapy. Clin Lymphoma Myeloma Leuk. 2019;19:763-775.e2.
- Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796-1805.
- Kabadi SM, Goyal RK, Nagar SP, et al. Treatment patterns, adverse events, and economic burden in a privately insured population of patients with chronic lymphocytic leukemia in the United States. Cancer Med. 2019;8:3803-3810.
- Mohan A, Yang K, Liu S, et al. Impact of atrial fibrillation on cardiovascular and economic outcomes in patients with chronic lymphocytic leukemia. Blood. 2021;138(suppl 1):4077-4078.
- Berger A, Simpson A, Bhagnani T, et al. Incidence and cost of major adverse cardiovascular events and major adverse limb events in patients with chronic coronary artery disease or peripheral artery disease. Am J Cardiol. 2019;123:1893-1899.
- O’Sullivan AK, Rubin J, Nyambose J, et al. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29:693-704.
- Matasar MJ, DaCosta Byfield S, Blauer-Peterson C, et al. What are the health care costs for chronic lymphocytic leukemia? J Clin Oncol. 2017;35(8 suppl):Abstract 15.
- Davids MS, Waweru C, Le Nouveau P, et al. Comparative efficacy of acalabrutinib in frontline treatment of chronic lymphocytic leukemia: a systematic review and network meta-analysis. Clin Ther. 2020;42:1955-1974.e15.
- Caldeira D, Alves D, Costa J, et al. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14:e0211228. doi: 10.1371/journal.pone.0211228.
- Mahida H, Gharia B, Ugoeke N, et al. Evaluation of cardiovascular adverse events associated with ibrutinib, venetoclax and idelalisib used in treatment of chronic lymphocytic leukemia. Circulation. 2018;138(suppl 1):A11835.
- Fischer K, Al-Sawaf O, Hallek M. Preventing and monitoring for tumor lysis syndrome and other toxicities of venetoclax during treatment of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2020;2020:357-362.
- Roeker LE, Fox CP, Eyre TA, et al. Tumor lysis, adverse events, and dose adjustments in 297 venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res. 2019;25:4264-4270.