Immune thrombocytopenia (ITP) is an autoimmune disorder in which platelet destruction and reduced platelet production lead to low platelet counts, or thrombocytopenia.1 ITP occurs in between 1 in 1000 and 1 in 10,000 pregnancies.2 A primary concern for patients with thrombocytopenia is the risk for bleeding. Severe bleeding can occur and is more common when the platelet count decreases to below 10 × 109/L.3
In patients with ITP during pregnancy, the fetus can be affected as well; autoantibodies causing thrombocytopenia can cross the placenta during pregnancy, leading to a risk for fetal thrombocytopenia.4 Other fetal complications, such as preterm delivery, are more common in pregnant women with ITP than in their pregnant counterparts without ITP.2,4 In addition, infection with SARS-CoV-2 (COVID-19) during pregnancy may be associated with an increased risk for adverse events, such as preeclampsia and preterm birth.5
The American Society of Hematology recommends the use of corticosteroids as a first-line therapy for patients with ITP, with the optional addition of rituximab in cases when inducing remission has high value.1 Concomitant intravenous (IV) immunoglobulin (IVIG) may also be used with steroids when more rapid recovery of platelet counts is needed.1 The second-line treatment options for ITP that persists for ≥3 months or that does not respond to corticosteroid therapy include the use of a thrombopoietin receptor agonist, rituximab, or splenectomy.1
For pregnant patients with ITP, the guidelines recommend treatment with corticosteroids or with IVIG; however, the guidelines do not provide treatment recommendations for pregnant patients with ITP that is refractory to first-line agents.1
The management of ITP during pregnancy is particularly important for meeting target platelet counts to administer anesthesia safely, perform a cesarean section, or complete a vaginal delivery. Between 2016 and 2018, hemorrhage was the fourth leading cause of pregnancy-related mortality in the United States.6
Although the first-line treatment for ITP in pregnancy has been established, the evidence for second-line treatment options, such as rituximab, thrombopoietin receptor agonist, and splenectomy, is limited primarily to case reports or case series.7-9 These reports show that second-line agents, such as rituximab and romiplostim, may be safe and effective during pregnancy for the treatment of ITP that is refractory to first-line agents.7-9
We report a case of a pregnant woman with ITP that was managed using all 3 second-line treatment modalities within the setting of COVID-19 infection, and the associated successful neonatal outcome. To the best of our knowledge, the treatment of concomitant ITP and active COVID-19 infection during pregnancy has not been previously published.
Case Report
A 19-year-old woman with ITP was admitted to our hospital with a platelet count of <2 × 109/L. Her chief complaint was a 2- to 3-day history of nausea and blood-tinged emesis, petechial rash, and a nosebleed on the day of presentation. She had been diagnosed with ITP on a previous admission and had received platelet transfusions, steroids, and IVIG.
After her initial diagnosis, the patient’s platelets recovered to 98 × 109/L by the time of her discharge; however, 7 days later her platelet count decreased again to 8 × 109/L.
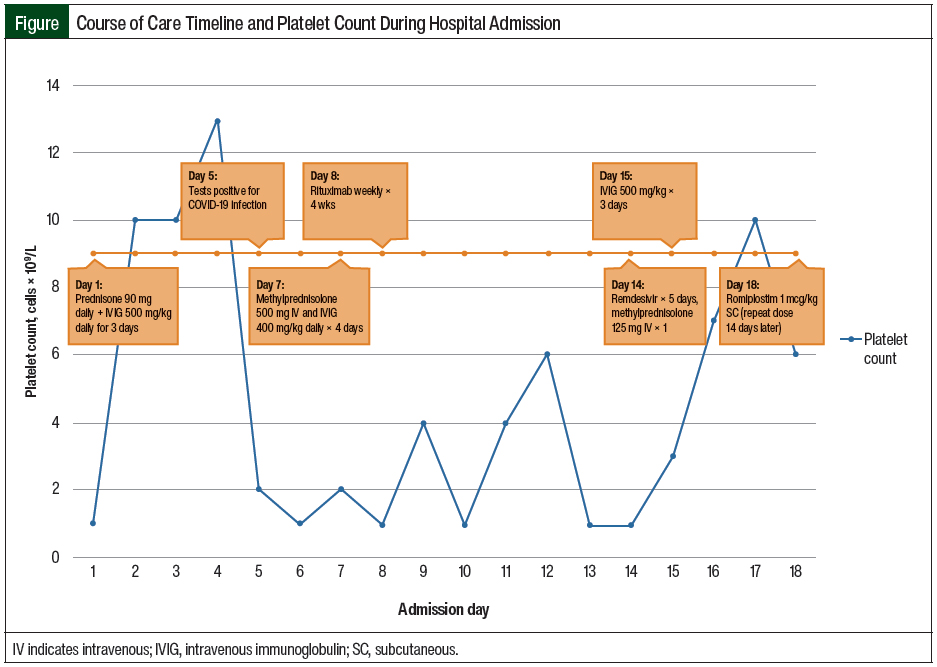
During the current admission, in addition to having a low platelet count, the patient was found to be pregnant, with a gestational age of 9 weeks and 4 days. Shortly after her admission, she complained of dyspnea, which led to the diagnosis of COVID-19. The Figure outlines the timeline of her care and platelet counts during her hospitalization.
On admission, the patient was prescribed initial treatment with prednisone 90 mg (approximately 1 mg/kg) daily and IVIG 500 mg/kg for 3 days. She continued to receive sulfamethoxazole 800 mg plus trimethoprim 160 mg 3 times weekly for Pneumocystis jirovecii pneumonia prophylaxis, which had been started during her previous admission.
Although splenectomy was considered for the patient, this was postponed until the second trimester to reduce the fetal risk associated with exposure to anesthesia during the first trimester.
On day 5 of her admission, the patient tested positive for COVID-19 and was administered remdesivir for 5 days, beginning on day 14 of her admission. The initiation of treatment with remdesivir was delayed after the patient had a positive COVID-19 test result: she had initially refused therapy for COVID-19 infection because of concerns related to the safety of the fetus.
The patient was unable to receive prophylactic anticoagulation per the facility’s COVID-19 protocol because of her severe thrombocytopenia, and she could not receive additional steroids for the treatment of COVID-19, because she was already receiving high-dose steroid therapy for ITP.
On day 7 of her admission, the patient’s platelets were 2 × 109/L; her steroid was converted to methylprednisolone 500 mg IV daily for 4 days, and treatment with IVIG was restarted at 400 mg/kg daily for 4 doses.
On day 8, rituximab (weekly for 4 doses) was initiated, because of persistent thrombocytopenia (<2 × 109/L) and a lack of response to treatment with steroids and IVIG. The patient’s platelets peaked at 6 × 109/L on day 12 and decreased to 2 × 109/L on day 13.
On day 14, a single dose of IV methylprednisolone 125 mg was administered, because her platelet count remained at 2 × 109/L, and on day 15, IVIG treatment was reinitiated at 500 mg/kg for 3 doses.
On day 18, having persistent thrombocytopenia despite receiving IVIG, steroids, and rituximab, the patient started receiving treatment with subcutaneous romiplostim 1 mcg/kg. She received a repeated dose of romiplostim 1 mcg/kg 14 days later (ie, day 32), for the treatment of persistently low platelet counts, while awaiting the full therapeutic effect of rituximab. Treatment with fostamatinib was not considered for our patient because of the facility’s formulary restrictions.
After discharge from the hospital, the patient underwent a scheduled splenectomy at our facility during the second trimester of pregnancy for the treatment of persistent thrombocytopenia. The fetus was monitored with fetal ultrasounds, and no morphologic abnormalities were noted.
The patient received an additional 2 doses of romiplostim 5 mcg/kg throughout the course of her pregnancy for the treatment of persistent severe ITP. The romiplostim dose increase was initiated because of the patient’s lack of response to previous lower doses of romiplostim. The 2 additional doses of romiplostim were given 21 days and 48 days after she received the first dose of romiplostim, and the platelet values just before administering these doses were 10 × 109/L and 4 × 109/L, respectively.
The patient’s pregnancy was noted to be complicated by fetal growth restriction, which was diagnosed at 23 weeks of gestation. The fetus was delivered via cesarean section at 37 weeks, without any additional notable maternal or fetal complications.
Discussion
The safety of therapies typically used for the second-line treatment of ITP has not been well-established in pregnant patients. Rituximab has not been reported to have teratogenic effects; however, fetal exposure to rituximab has been connected to prolonged fetal B-cell lymphopenia, which may require a delay of routine vaccinations in affected neonates.2,9 In addition, there have been conflicting reports as to whether treatment with rituximab before infection with COVID-19 may be associated with increased risk for more severe COVID-19 symptoms or a protracted course of the infection because of B-cell depletion.10,11
In a study comparing COVID-19 outcomes among patients with rheumatoid arthritis, those who received rituximab before infection were more likely to require hospitalization and had an increased risk for mortality compared with patients who received alternative therapies.12 By contrast, a separate study of patients who had received rituximab before COVID-19 infection showed no differences in the rate of hospitalization, intensive care unit (ICU) admission, or mortality related to the timing of their last rituximab infusion.10
Our patient had mild COVID-19 symptoms at the time of her positive COVID-19 PCR (polymerase chain reaction) test, which led the team to recommend the use of rituximab despite her infection. After receiving rituximab, her COVID-19 symptoms did not worsen, and she did not require admission to the ICU or additional escalation of care related to COVID-19.
Overall, pregnant women were initially excluded from clinical trials that evaluated the use of remdesivir treatment in patients with COVID-19 infection; however, pregnant women were able to receive remdesivir beginning in March 2020 through a compassionate-use program.13 A report of the outcomes in the first 86 pregnant women who received compassionate-use remdesivir treatment described that therapy as safe, well-tolerated, and associated with high rates of clinical recovery from COVID-19.13 These compassionate-use data supported the use of remdesivir treatment safely in our patient.
Romiplostim, a thrombopoietin receptor agonist, lacks robust data about human fetal risk, but it has been shown to increase fetal mortality in animal models.14 In addition, romiplostim is associated with a risk for thromboembolic complications,5 which when paired with active COVID-19 increased our concern for clotting events. However, the thromboembolic risk associated with romiplostim is related to the increasing platelet count and thrombocytosis, and is minimized by following the recommended dose adjustments.5
A multicenter, retrospective study of 15 pregnant women with ITP who were exposed to thrombopoietin receptor agonist therapy reported no cases of maternal thromboembolic events, thrombocytopenia cases in 6 of 17 neonates, and only 1 case of neonatal thrombocytosis.15 An overall response to therapy was seen in 10 of the 15 women.15
In a case report describing the use of romiplostim in 2 pregnant women with ITP, the first woman had uterine atony and hemorrhage after the delivery of a healthy neonate, and the second woman’s delivery was complicated by preeclampsia, with the neonate having a cranial hemorrhage related to fetal ITP.8
An additional report of 2 patients receiving romiplostim during pregnancy describes maternal adverse events, such as preeclampsia, heavy postpartum bleeding, and uterine atony, as well as fetal adverse events, including hyperbilirubinemia and hypoglycemia.16 These adverse events are reported within the setting of observational case reports16; therefore, it is difficult to be certain whether these events were attributable to romiplostim exposure or were a complication of maternal ITP.
We discussed these potential adverse events, as well as other yet unknown adverse events with the patient, and ultimately it was decided that the benefit of potentially treating her refractory ITP outweighed the potential risk for the fetus.
The selection of romiplostim over eltrombopag as the thrombopoietin receptor agonist for our patient was primarily driven by our facility’s formulary; however, a subsequent review of the available literature showed that treatment with eltrombopag during pregnancy may be more likely to cause intrauterine growth restriction and low fetal birth weight compared with the use of romiplostim.17,18
A retrospective, matched-cohort study of 195 patients who underwent splenectomy for ITP during the second trimester of pregnancy showed no significant difference in adverse events compared with 975 nonpregnant patients undergoing splenectomy for ITP.19 Although these results are encouraging to claim the potential safe use of thrombopoietin receptor agonists and splenectomy during pregnancy, further studies with a larger sample size are needed to confirm the safety of these interventions.
Conclusion
This case presents a complex treatment of ITP during pregnancy that was complicated by concomitant COVID-19 infection. To the best of our knowledge, there are no available studies or other cases describing the use of rituximab in a patient with active COVID-19 infection, and there is sparse literature describing the use of remdesivir and romiplostim in pregnant patients. The ability to generalize from this case report to other patients is limited because the patient received romiplostim intermittently rather than on a weekly dosing schedule.
This case report adds to the growing literature describing the use of second-line interventions to manage ITP during pregnancy with concomitant COVID-19 infection. Further studies are needed to confirm the causality between treatment with thrombopoietin receptor agonists and the reported fetal adverse events.
Author Disclosure Statement
Dr Rezac and Dr Wittig have no conflicts of interest to report.
References
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829-3866. Erratum in: Blood Adv. 2020;4:252.
- Cines DB, Levine LD. Thrombocytopenia in pregnancy. Hematology Am Soc Hematol Educ Program. 2017;2017:144-151.
- Arnold DM. Bleeding complications in immune thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2015;2015:237-242.
- Fogerty AE. Thrombocytopenia in pregnancy: mechanisms and management. Transfus Med Rev. 2018;32:225-229.
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540-E548.
- Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. Updated June 22, 2022. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Accessed January 30, 2023.
- Chon AH, Chan R, Lee RH, et al. Multidrug therapy for refractory immune thrombocytopenia in pregnancy. Obstet Gynecol. 2020;135:723-727.
- Chua SJ, Morton MR, Svigos J, et al. Use of romiplostim in pregnancy for refractory idiopathic thrombocytopenic purpura: two case reports with maternal and fetal outcomes and literature review. Obstet Med. 2020;13:45-50.
- Das G, Damotte V, Gelfand JM, et al. Rituximab before and during pregnancy: a systematic review, and a case series in MS and NMOSD. Neurol Neuroimmunol Neuroinflamm. 2018;5:e453. doi: 10.1212/NXI.0000000000000453.
- Levavi H, Lancman G, Gabrilove J. Impact of rituximab on COVID-19 outcomes. Ann Hematol. 2021;100:2805-2812.
- Daoussis D, Leonidou L, Kalogeropoulou C, et al. Protracted severe COVID-19 pneumonia following rituximab treatment: caution needed. Rheumatol Int. 2021;41:1839-1843.
- Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137-1146.
- Burwick RM, Yawetz S, Stephenson KE, et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis. 2021;73:e3996-e4004.
- Nplate (romiplostim) for injection, for subcutaneous use [prescribing information]. Amgen; February 2022. www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Nplate/nplate_pi_hcp_english.pdf. Accessed January 30, 2023.
- Michel M, Ruggeri M, Gonzalez-Lopez TJ, et al. Use of thrombopoietin receptor agonists for immune thrombocytopenia in pregnancy: results from a multicenter study. Blood. 2020;136:3056-3061.
- Samuelson BT, Baumann Kreuziger L, Gernsheimer T. Use of romiplostim for refractory primary immune thrombocytopenia during pregnancy. Clin Obstet Gynecol Reprod Med. February 2017;3. doi: 10.15761/COGRM.1000172.
- Eslick R, McLintock C. Managing ITP and thrombocytopenia in pregnancy. Platelets. 2020;31:300-306.
- Howaidi J, AlRajhi AM, Howaidi A, et al. Use of thrombopoietin receptor agonists in pregnancy: a review of the literature. Hematol Oncol Stem Cell Ther. 2022;15:1-6.
- Bleau N, Czuzoj-Shulman N, Spence AR, Abenhaim HA. Safety of splenectomy during pregnancy. J Matern Fetal Neonatal Med. 2017;30:1671-1675.