Acute lymphoblastic leukemia (ALL) is a cancer of the blood and bone marrow that affects the white blood cells (lymphocytes).1 In 2017, 5970 new patients were estimated to be diagnosed with ALL and 1440 individuals to die from this disease.2 ALL is diagnosed most often in children, adolescents, and young adults, with a median age of 15 years at diagnosis.2
The 5-year survival rates for ALL increased by 6.7% between 1990-1994 and 2000-2005 for patients who received treatment.3 However, for patients whose prognosis is poor because of relapsed (particularly after a second complete remission) or refractory disease, challenges persist and treatment options are limited.4,5
The treatment for ALL may include chemotherapy, targeted therapy (for Philadelphia chromosome–positive ALL), radiation therapy, stem-cell transplantation, and enrollment in clinical trials.1
Kymriah First Gene Therapy Approved by the FDA
On August 30, 2017, the US Food and Drug Administration (FDA) approved tisagenlecleucel (Kymriah; Novartis), a CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapy, for the treatment of patients aged ≤25 years with B-cell precursor ALL that is refractory, in second relapse, or later relapse.6,7 Tisagenlecleucel is the first gene therapy to receive FDA approval in the United States.6
Commenting on this approval, FDA Commissioner Scott Gottlieb, MD, said, “We’re entering a new frontier in medical innovation with the ability to reprogram a patient’s own cells to attack a deadly cancer. New technologies such as gene and cell therapies hold out the potential to transform medicine and create an inflection point in our ability to treat and even cure many intractable illnesses.”6
The FDA approved tisagenlecleucel with a Risk Evaluation and Mitigation Strategy (REMS) program, because of the associated risk for cytokine release syndrome (CRS) and neurologic adverse events with this immunotherapy.6 In addition, the FDA required that clinicians who dispense tisagenlecleucel are specially certified on prescribing, dispensing, and administering this medication and on recognizing and managing CRS and the associated neurologic adverse reactions.6
Mechanism of Action
CD19 is a protein that is expressed on B-cells in leukemia and other cancers.8 Tisagenlecleucel is a CD19-directed, genetically modified, autologous, CAR T-cell immunotherapy that is customized using the patient’s peripheral blood T-cells, which are collected via a leukapheresis procedure.7 The patient’s T-cells are reprogrammed with a transgene encoding a CAR. These reprogrammed CAR T-cells transmit a signal that identifies, targets, and eliminates CD19-expressing malignant and normal cells.7 After the patient’s cells are modified and multiplied, they are infused back into the patient’s bloodstream, where they expand further, activating the elimination of cancer cells and the persistence of the tisagenlecleucel.7,8
Dosing and Administration
Tisagenlecleucel is for autologous and for intravenous use only. Patients should be premedicated with acetaminophen and an H1-antihistamine. The availability of tocilizumab (Actemra) must be confirmed before administering tisagenlecleucel, in case CRS occurs.7
The dosing of tisagenlecleucel is based on the patient’s number of CAR-positive viable T-cells, and the patient’s weight, as outlined in the prescribing information. A single dose of tisagenlecleucel may contain up to 2.5 × 108 CAR-positive viable T-cells that are suspended in a single patient-specific infusion bag. The Certificate of Analysis should be referred to for the actual cell count. The infusion bag volume ranges from 10 mL to 50 mL.7
Pivotal Clinical Trial
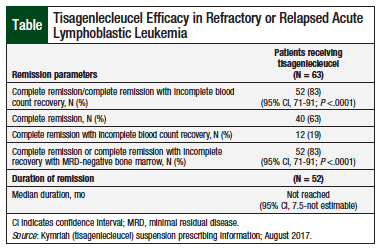
The FDA approval of tisagenlecleucel was based on results from the ELIANA study, an open-label, single-arm, phase 2 clinical trial.7,9 Of the 63 evaluable pediatric and young adult patients, 35 were males and 28 were females (median age, 12 years; range, 3-23 years).7
In ELIANA, 83% of patients who received tisagenlecleucel therapy had complete remission or complete remission with incomplete blood count recovery within 3 months after infusion (Table).7 All the patients who had complete remission or complete remission with incomplete blood count recovery were negative for minimal residual disease, and 12% of them had stem-cell transplant within 6 months, while still in remission.7 The median duration of complete remission or complete remission with incomplete blood count recovery was not reached by the time of the analysis.7
Adverse Reactions
The most common (>20%) all-grade adverse reactions reported with tisagenlecleucel are CRS (79%), hypogammaglobulinemia (43%), infections–pathogen unspecified (41%), pyrexia (40%), decreased appetite (37%), headache (37%), encephalopathy (34%), hypotension (31%), bleeding episodes (31%), tachycardia (26%), nausea (26%), diarrhea (26%), vomiting (26%), viral infectious disorders (26%), hypoxia (24%), fatigue (22%), acute kidney injury (22%), and delirium (21%).7
The most common grade ≥3 events are CRS (49%), hypotension (22%), hypoxia (18%), pyrexia (15%), viral infectious disorders (18%), infections–pathogen unspecified (16%), decreased appetite (15%), bacterial infectious disorders (13%), acute kidney injury (13%), encephalopathy (10%), and pulmonary edema (10%).7
Drug Interactions
HIV and the lentivirus used to make tisagenlecleucel have limited, short spans of identical genetic material. Therefore, some commercial HIV nucleic acid tests may yield false-positive results in patients who receive tisagenlecleucel.7
Warnings and Precautions
Tisagenlecleucel includes a boxed warning about the risk for fatal or life-threatening CRS associated with this therapy. Patients with active infection or inflammatory disorders should not use tisagenlecleucel.7
The boxed warning also cautions about the potential for severe or life-threatening neurologic reactions with tisagenlecleucel. Patients should be monitored for neurologic adverse events. Tisagenlecleucel is only available through the restricted Kymriah REMS program.7
Patients should also be monitored for hypersensitivity reactions and for signs of infection.7
Cytopenias may occur for several weeks after infusion; prolonged neutropenia can increase the risk for infection.7
Patients with complete remission after tisagenlecleucel therapy may have hypogammaglobulinemia or agammaglobulinemia. Immunoglobulin levels should be monitored and assessed in newborns of mothers who receive tisagenlecleucel therapy.7
Recurrence of leukemia or secondary malignancies may occur with tisagenlecleucel.7
Patients should refrain from driving and engaging in hazardous work or activities, such as operating heavy or potentially dangerous equipment, for at least 8 weeks after receiving tisagenlecleucel.7
Use in Specific Populations
The safety and efficacy of tisagenlecleucel have been established in young patients (aged 3 years to <19 years) in the ELIANA study, which showed no differences in efficacy or safety between the different age subgroups. The data are insufficient to establish the safety and efficacy of tisagenlecleucel in older patients aged ≥65 years.7
Conclusion
Tisagenlecleucel is the first gene therapy to receive FDA approval in the United States. This approval brings a new, innovative immunotherapy for the treatment of young patients with relapsed or refractory B-cell precursor ALL.
References
- Mayo Clinic staff. Acute lymphocytic leukemia. January 28, 2016. www.mayoclinic.org/diseases-conditions/acute-lymphocyticleukemia/basics/definition/con-20042915. Accessed October 24, 2017.
- National Cancer Institute. SEER cancer stat facts: leukemia – acute lymphocytic leukemia (ALL). https://seer.cancer.gov/statfacts/html/alyl.html. Accessed October 24, 2017.
- Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1663-1669.
- Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017-4023. Erratum in: Blood. 2016;128:1441.
- Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129-136.
- US Food and Drug Administration. FDA approval brings first gene therapy to the United States. August 30, 2017. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm. Accessed October 20, 2017.
- Kymriah (tisagenlecleucel) suspension [prescribing information]. East Hanover, NJ: Novartis; August 2017.
- Leukemia & Lymphoma Society. Chimeric antigen receptor (CAR) T-cell therapy. www.lls.org/treatment/types-of-treatment/immunotherapy/chimericantigen-receptor-car-t-cell-therapy. Accessed December 11, 2017.
- Buechner J, Grupp SA, Maude SL, et al. Global registration trial of efficacy and safety of CTL019 in pediatric and young adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL): update to the interim analysis. Presented at the Congress of the European Hematology Association; June 22-25, 2017; Madrid, Spain.