Anemia is an independent prognostic factor for survival in patients with certain cancers and has been associated with reduced survival times.1 The prevalence of iron-deficiency anemia is not well measured in patients with cancer but is estimated to range from 5% to 25%, depending on the patient’s cancer type.1
Potential treatments for iron-deficiency anemia include oral or intravenous (IV) iron formulations. The IV iron formulations that are currently available in the United States include iron sucrose, low-molecular-weight iron dextran, ferrous gluconate, ferric carboxymaltose, iron isomaltoside, and ferumoxytol.2 IV iron administration is used when oral iron formulations are ineffective, poorly tolerated (usually as a result of gastrointestinal side effects), or poorly absorbed, or when blood transfusions are inappropriate.3 In patients who may require IV iron supplementation, it is important to weigh the risks versus the benefits (such as reducing the previous poor tolerance or poor absorption of oral iron) based on individual patient factors. Allergic reactions or hypersensitivities to IV iron usually occur with the first dose, but they can arise despite having no reaction with test doses and tolerance of previous iron infusions.4
The potential for a reaction with these various IV iron formulations differs based on the iron product choice and patient-specific factors. Assessing patient-specific factors is important when risk stratifying patients, which may guide treatment decisions, including the use of premedication, the infusion rate, and the intervals of observation during and after receiving an iron infusion. Therefore, it is crucial to exercise caution with every iron infusion to provide optimal patient care. This overview outlines the current evidence-based recommendations for managing the different severities of iron infusion reactions and highlights the means to reduce the risk for or prevent IV iron hypersensitivity reactions.
Symptom Overview
Fishbane-type reactions are a specific type of transient reaction to IV iron products.5 Fishbane-type reactions usually consist of acute chest and back tightness and joint pain without severe symptoms, such as hypotension, wheezing, stridor, or laryngeal edema.3,5 Fishbane-type reactions occur in approximately 1 in 100 patients who receive IV iron products.3,5 The pathogenesis of Fishbane-type reactions has not been thoroughly investigated.3
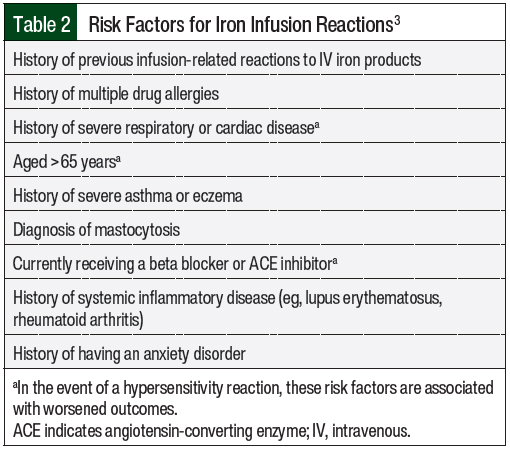
Reactions that do not fall under Fishbane-type categorization are further characterized by the severity and time to the resolution of symptoms. For mild reactions, the symptoms include flushing, urticaria, itching, sensation of heat, slight chest tightness, hypertension, joint pain, and back pain.3 The symptoms of moderate reactions include the symptoms listed under mild reactions and transient cough as the initial features, but they may also include chest tightness, shortness of breath, tachycardia, and hypotension (Table 1).3 Last, severe reactions include the symptoms of wheezing, stridor, periorbital edema, cyanosis, confusion, loss of consciousness, and cardiac and/or respiratory arrest.3 It is critical to understand and assess the severity of these reactions and Fishbane-type reactions to properly manage the hypersensitivity reactions.
ETIOLOGY
The results of a study of 11,130 patients who had 195 infusion reactions to the IV iron formulations of iron polymaltose, ferric carboxymaltose, or ferric derisomaltose showed adverse event rates of 2.2%, 0.4%, and 0.5%, respectively.5 Of the 195 adverse hypersensitivity reactions among the 13,509 iron infusions, 59 (30%) were Fishbane-type, 70 (36%) were mild, 48 (25%) were moderate, and 7 (3%) were severe. All of the 59 patients who had Fishbane-type reactions had truncal myalgia and 12 of the 59 patients had flushing. There were no patient deaths. Delayed reactions, which were defined as occurring after the completion of the iron infusion, occurred in 11 (6%) patients.5 The rarity of iron infusion reactions may contribute to the suboptimal management of hypersensitivity reactions.
Iron infusion reactions are hypothesized to be caused by the 2 main mechanisms of immunoglobulin E–mediated response and complement activation–related pseudoallergy resulting from nanoparticles in the iron product formulations.6 Both of these hypothesized pathways converge at the activation of mast cells and basophils, which leads to the secretion of histamine, thromboxane, leukotrienes, and platelet-activating factor.6 The release of these factors leads to symptoms such as bronchospasm, laryngeal edema, tachycardia, hypotension or hypertension, hypoxia, and the loss of consciousness.6 The potential hypersensitivity reactions to iron infusions are divided into the 4 main categories of Fishbane-type, mild, moderate, and severe. The Fishbane-type reaction is thought to be caused by labile and free iron.7 Labile iron is weakly bound iron within a nanoparticle formulation that can readily react chemically or mobilize as a result of “iron complex forming agents, such as proteins in blood plasma.”7
The rate of infusion is an important factor that impacts the incidence of iron infusion–related reactions, which is also a result of increased labile free iron.7 Another suggested reason for iron infusion–related reactions is the body’s ability to clear anaphylatoxins from the blood versus the rate of infusion into the body.6,8 Therefore, the prevention of infusion reactions can be attempted via reducing the infusion rates. In patients who are rechallenged with iron infusions, reducing the infusion rate may also prevent the recurrence of infusion-related adverse reactions.
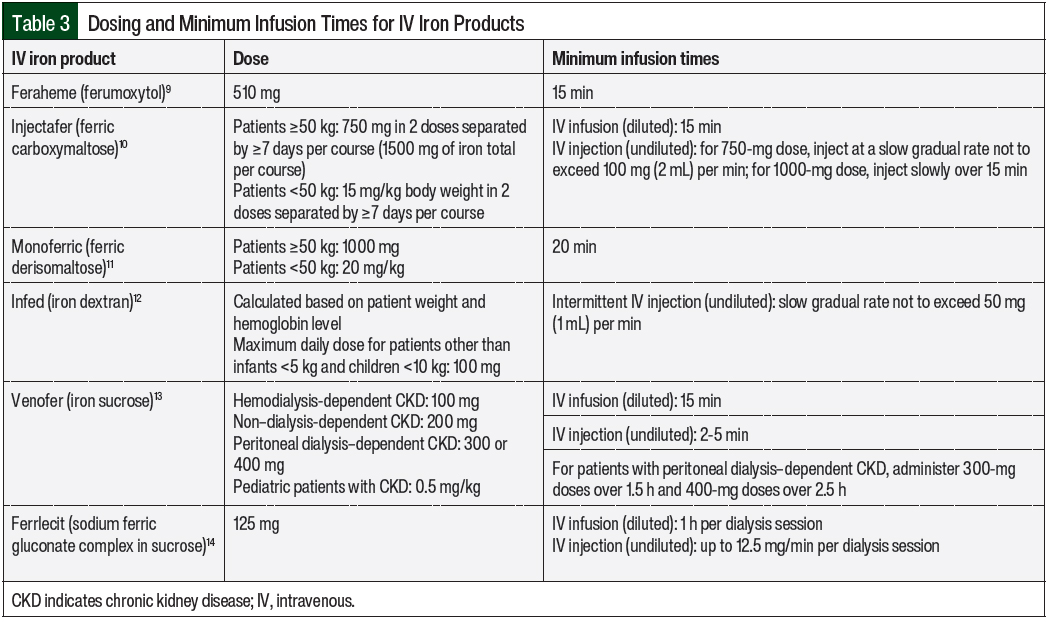
The risk factors for iron infusion reactions, including those associated with worse outcomes, are shown in Table 2.3
PREVENTION AND TREATMENT OPTIONS
IV iron products should only be administered when trained personnel and rescue therapies are immediately available for the treatment of anaphylaxis and/or other hypersensitivity reactions.4 As best practice, all patients should be assessed for infusion reactions every 15 minutes during an infusion and should be observed for at least 30 minutes after an infusion.3 For any of the 4 reaction subtypes, it is crucial to pause or stop the infusion at the first signs of an infusion reaction. Steps for the management of infusion reactions are outlined in Table 1.
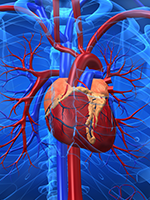
The minimum infusion rates for iron products are formulation-specific and are included in the prescribing information for each iron product (Table 3).9-14 Patients who have at least 1 risk factor for infusion reactions may be considered for a cautious infusion rate of 50% of the standard rate until tolerance is shown (ie, after 10-15 minutes).3 For patients who tolerate an iron product at 50% of the standard administration rate, the standard administration rate should be considered for the IV iron agent as outlined by its prescribing information. Patients who have multiple risk factors for hypersensitivity reactions may be considered for dosing at 10% of the standard administration rate.3 Patients who have a higher risk for infusion reactions should be observed for 30 to 60 minutes after the end of the iron infusion.3 These factors can be used to assess the patients’ additional options for risk mitigation with the use of IV iron.
A test dose is recommended for IV iron dextran before the administration of a full therapeutic dose.12 Test doses are not recommended for other IV iron products that are commercially available in the United States.9-11,13,14 However, test doses may provide false reassurance. A 2013 European Medicines Agency report concluded that small test doses of an IV iron product may not reliably predict how a patient will respond to receiving a full dose of the iron product.4 Adverse reactions may still occur in patients who have not had a reaction after receiving a test dose of an IV iron product. Therefore, caution is warranted with every dose of IV iron, even if previous administrations or test doses have been well tolerated.
The use of premedications before iron infusions is lacking robust data. Notably, the following recommendations are only for patients with ≥1 risk factor in Table 1 or with ≥1 of the following comorbidities: asthma, inflammatory arthritis, or having more than 1 drug allergy. For patients with these comorbidities, premedication with a corticosteroid can be considered and should be administered 30 to 60 minutes before the initiation of an iron infusion.15
Diphenhydramine is not recommended as a premedication nor as a rescue medication for Fishbane-type reactions.3,16 Diphenhydramine can worsen Fishbane-type reactions as a result of documented adverse events, including hypotension along with known adverse events of diphenhydramine, such as dizziness, thickening of bronchial secretions, flushing, irritability, increased risk of falls, and anticholinergic symptoms (eg, dry mouth).16 In addition to Fishbane-type reactions, diphenhydramine is not recommended for use in patients who have had moderate, severe, or anaphylactic hypersensitivity reactions, because the use of H1 antihistamines and vasopressors may convert mild, nonallergic infusion reactions to more serious adverse reactions.16 These more serious adverse reactions can be erroneously attributed to the IV iron product and may complicate future decisions on iron replenishment.16
The management strategies for iron infusion reactions are based on the type and severity of the infusion reaction.3 The symptoms of the various reaction types as well as management strategies for these reactions are shown in Table 1. For mild hypersensitivity reaction or Fishbane-type reaction, the infusion should be stopped and the patient should be observed. If the patient is stable after 15 minutes of the infusion being paused, the iron infusion should be completed at ≥50% of the initial rate. If the patient becomes unstable or if the symptoms worsen, the reaction should be treated as a moderate or severe reaction as appropriate.3
For moderate hypersensitivity reactions, the infusion should be stopped immediately and a volume load (eg, 500 mL of 0.9% saline) or an IV corticosteroid (eg, hydrocortisone 100-500 mg) can be administered.3 If the patient is unstable or if the symptoms worsen, the reactions should be treated as a severe reaction.3
In patients with severe reactions, the iron infusion should be stopped immediately and a rapid response protocol should be initiated including the administration of epinephrine, corticosteroids, vasopressors, and other medications as indicated based on practice site–specific protocols.3 All sites that administer IV iron products should have proper training, staffing, and resources to manage these severe reactions.4 If the patient is unstable or if their symptoms worsen after these protocol measures, he or she should be transferred to an acute or intensive care setting.3 These patients should be observed for 4 to 24 hours after the infusion is attempted. The management of patients who have a severe reaction should be based on clinical judgement and appropriate resuscitation measures.3
A trial that evaluated the prevalence of iron infusion reactions monitored the outcomes of rechallenging patients with different formulations of IV iron.5 Of 69 patients, 9 (13%) were rechallenged with the same formulation (iron polymaltose) and 60 (87%) were rechallenged with an alternative formulation (ferric carboxymaltose).5 The researchers reported a 98% success rate when switching patients to an alternative iron formulation.5 Of note, iron polymaltose is not available in the United States. In all, 61% of the patients received premedication with an antihistamine, a corticosteroid, or both, and only 3 patients were rechallenged at a slower rate of iron infusion than they initially received.5
Another study assessed the use of sodium ferric gluconate complex (SFGC) in patients who were undergoing hemodialysis and who had received iron dextran.17 Of 143 patients who were intolerant to treatment with iron dextran, 3 patients (2.1%) were intolerant of treatment with SFGC. Of 2194 patients who previously tolerated treatment with iron dextran, only 7 (0.3%) were intolerant of treatment with SFGC.17 Patients with a history of iron dextran intolerance had a 7-fold higher rate of reactions to treatment with SFGC compared with iron dextran–tolerant patients (P=.02).17 Furthermore, the investigators determined that SFGC reactions may actually be a “pseudoallergy” that results from low incidence (ie, at a similar incidence to reactions with placebo), low mast cell activation, and patient histories including other drug allergies that may contribute.17
As indicated by these data, cross tolerance or sensitivity can be highly dependent on patient-specific factors, such as a history of drug sensitivities, a history of anaphylactoid reactions to previous iron infusions, and an overall history with iron infusions. Overall, these data provide important insight on the significance of documenting reactions and outcomes as treatment teams attempt to adequately supplement iron after hypersensitivity reactions.17
As with any hypersensitivity or allergic reaction, it is critical to properly document the reaction and the result. This is especially important in the setting of iron infusion reactions that result from differences in the formulation options. Documentation should include the severity of the reaction, the previous IV iron formulations the patient has received, the risk factors, the initial symptoms, symptom progression, interventions and subsequent patient responses, and the timing of the symptoms and their resolution.3 These details can help guide future therapy decisions, and the outcomes can serve as a guide for providers if they wish to rechallenge patients.
CONCLUSION
Infusion reactions are rare with IV iron products, and the management of these reactions depends on their type and severity. An initial risk stratification can help identify if a patient is an ideal candidate for treatment and may help determine the best approach to administer IV iron. IV iron products are important supportive-care agents in the oncologic setting according to evidence that anemia is an independent negative prognostic factor for survival in patients with certain cancers. Increased knowledge and experience with identifying the risks and managing infusion reactions can help improve evidence-based care of patients who receive IV iron products.
Author Disclosure Statement
Dr Laza, Dr Keisler, Dr Handy, and Dr Dickens have no conflicts of interest to report.
REFERENCES
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214-2221.
- Ning S, Zeller MP. Management of iron deficiency. Hematology Am Soc Hematol Educ Program. 2019;2019:315-322.
- Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica. 2014;99:1671-1676.
- European Medicines Agency. New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines. September 13, 2013. Accessed October 12, 2023. www.ema.europa.eu/en/documents/referral/intravenous-iron-containing-medicinalproducts-article-31-referral-new-recommendations-manage-risk_en.pdf.
- Stojanovic S, Graudins LV, Aung AK, et al. Safety of intravenous iron following infusion reactions. J Allergy Clin Immunol Pract. 2021;9:1660-1666.
- Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106-121.
- Jahn MR, Andreasen HB, Fütterer S, et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm. 2011;78:480-491.
- Szebeni J, Baranyi L, Sávay S, et al. Complement activation–related cardiac anaphylaxis in pigs: role of C5a anaphylatoxin and adenosine in liposome-induced abnormalities in ECG and heart function. Am J Physiol Heart Circ Physiol. 2006;290:H1050-H1058.
- Feraheme (ferumoxytol) injection, for intravenous use [prescribing information]. AMAG Pharmaceuticals; June 2022.
- Injectafer (ferric carboxymaltose) injection, for intravenous use [prescribing information]. American Regent; May 2023.
- Monoferric (ferric derisomaltose) injection, for intravenous use [prescribing information]. Pharmacosmos Therapeutics; February 2022.
- Infed (iron dextran) injection, for intravenous or intramuscular use [prescribing information]. Allergan USA; April 2021.
- Venofer (iron sucrose) injection, for intravenous use [prescribing information]. American Reagent; June 2022.
- Ferrlecit (sodium ferric gluconate complex in sucrose) injection, for intravenous use [prescribing information]. Sanofi-Aventis; March 2022.
- Auerbach M, Chaudhry M, Goldman H, Ballard H. Value of methylprednisolone in prevention of the arthralgia-myalgia syndrome associated with the total dose infusion of iron dextran: a double blind randomized trial. J Lab Clin Med. 1998;131:257-260.
- Gómez-Ramírez S, Shander A, Spahn DR, et al. Prevention and management of acute reactions to intravenous iron in surgical patients. Blood Transfus. 2019;17:137-145.
- Coyne DW, Adkinson NF, Nissenson AR, et al. Sodium ferric gluconate complex in hemodialysis patients. II. Adverse reactions in iron dextran-sensitive and dextran-tolerant patients. Kidney Int. 2003;63:217-224.