Methotrexate (MTX) is an antimetabolite chemotherapeutic agent. It is specifically an antifolate and binds and inhibits dihydrofolate acid reductase, interfering with purine and thymidylic acid synthesis, and ultimately preventing DNA synthesis, repair, and cellular replication.1 In general, high-dose MTX (HD-MTX) refers to MTX doses higher than 500 mg/m2.2 The use of an HD-MTX–based regimen is suggested by the National Comprehensive Cancer Network guidelines as a treatment for diffuse large B-cell lymphoma as well as primary central nervous system (CNS) lymphoma.3,4
The management and prevention of the adverse events associated with the use of HD-MTX requires interdisciplinary collaboration between clinical pharmacy, hematology/oncology service, and the primary medicine/nursing team in the hospital treatment unit. HD-MTX has the potential to cause gastrointestinal, hematologic, hepatic, neurologic, pulmonary, renal, and dermatologic adverse events, as well as multiple others.1 In patients with normal renal function, HD-MTX can be safely administered with the use of several supportive-care strategies employed before and after administration.2 Before HD-MTX is given, a patient should be evaluated for any fluid pockets and effusions that may delay the elimination of MTX.1 The primary elimination method for MTX is renal excretion enhanced by vigorous hydration and urine alkalinization.1 During the clearance of HD-MTX, a leucovorin rescue is started 24 hours after the start of MTX infusion and given until the MTX level is <0.05 µmol/L.5 During this time, urine output, urine pH, fluid balance, and renal function are closely monitored. After HD-MTX, MTX levels are monitored daily by a homogeneous enzyme immunoassay and subsequent spectrophotometry.6
We present a case of false-positive delay in MTX clearance in a patient undergoing his first cycle of HD-MTX despite adequate pre- and postmanagement to prevent adverse events and enhance MTX urine elimination.
Case Report
A man aged 74 years presented to the Veterans Affairs (VA) Boston Healthcare System with altered mental status and had multiple enhancing brain lesions with vasogenic edema status after receiving high-dose steroids. A stereotactic brain biopsy demonstrated high-grade large B-cell lymphoma that was non–germinal cell and CD10-negative. On fluorescence in situ hybridization analysis, BCL6 rearrangement was positive, but there was no rearrangement of MYC or IGH/BCL2. Computed tomography scan showed few subcentimeter, right lower lobe pulmonary nodules; mesenteric, periportal, and right external iliac lymph nodes; and right renal hypodense lesions measuring up to 2.5 cm. A biopsy of the enlarged iliac nodes was consistent with grade 1 or 2 CD10-positive follicular lymphoma.
The decision by the hematology/oncology team was to initiate HD-MTX (3.5 g/m2) to try to rapidly control his CNS disease. Before his treatment, the patient’s medication list was evaluated by an oncology pharmacy specialist for any interactions with MTX that may reduce the clearance or increase adverse events. The only interacting medication was pantoprazole, which was discontinued before his cycle. The other notable medication was hydrochlorothiazide 25 mg plus triamterene 37.5 mg orally once daily for blood pressure control, but this was not noted to interact with the MTX clearance at the time of pharmacy review. A single case report was noted via tertiary database interaction checker, regarding possible increased MTX adverse events in combination with triamterene.7 However, this theoretical interaction was based on a low level of evidence, and there was no recommendation for the discontinuation of hydrochlorothiazide 25 mg plus triamterene 37.5 mg given the supportive care he would receive after infusion. A chest x-ray was also conducted before starting treatment with HD-MTX to rule out any fluid pockets that may result in third spacing and that would delay the elimination of MTX.
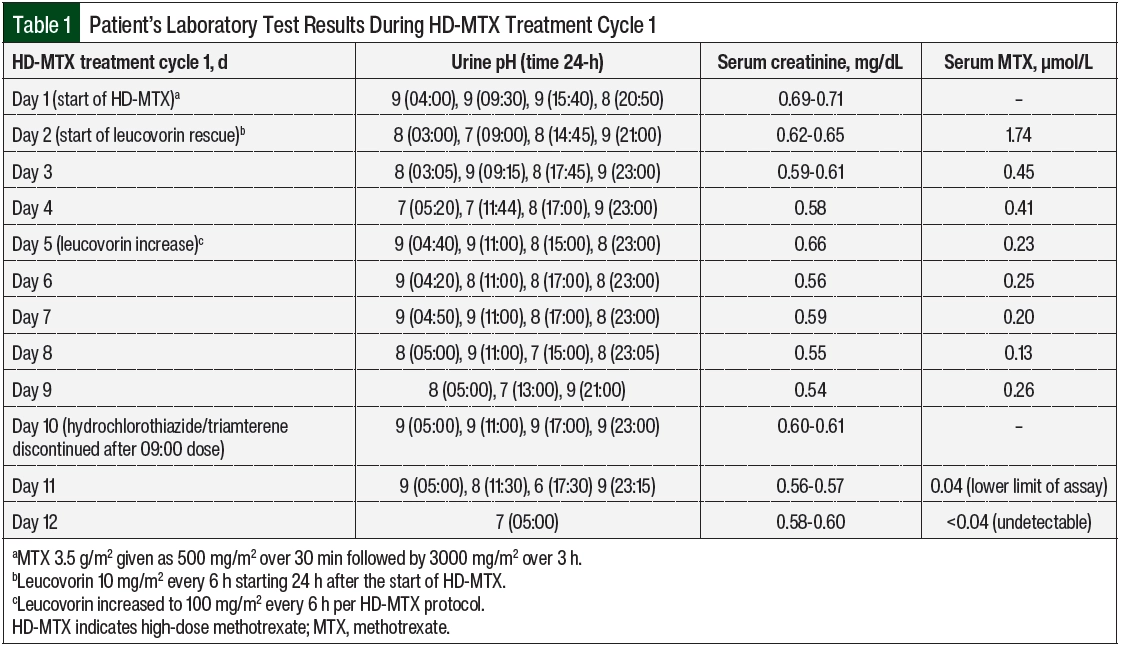
Per VA Boston Healthcare System’s HD-MTX protocol, the patient’s urine was alkalized to a pH of >7 starting ≥12 hours before his treatment cycle. On day 1 of this cycle, with a urine pH of 9 and serum creatinine within normal limits (Table 1), MTX 3.5 g/m2 was infused as 500 mg/m2 over 30 minutes followed by 3000 mg/m2 over 3 hours. There were no noted complications with his supportive medications or chemotherapy administered on day 1. Per protocol, leucovorin 10 mg/m2 was started on day 2, approximately 24 hours after the start of the HD-MTX. The patient’s renal function and urine pH remained stable, and the 24- and 48-hour serum MTX levels indicated appropriate and expected clearance from 1.7 to 0.45 µmol/L. However, on day 4, the serum MTX level remained relatively unchanged at 0.23 µmol/L. The patient’s urine pH had decreased to 7, which was thought to be a possible explanation for the sustained MTX level. The patient also had mild transaminitis, which is a common reversible change after treatment with HD-MTX.2
On day 5, the MTX level remained at 0.25 µmol/L, and the leucovorin was increased to 100mg/m2 every 6 hours per the HD-MTX protocol to prevent bone marrow adverse events. Another chest x-ray was ordered to check for a third space effusion as a possible cause for delayed elimination. No effusions were noted, and the patient’s urine pH and serum creatinine had remained optimized. On days 6 through 8, the serum MTX level remained plateaued at >0.1 µmol/L (target, <0.05 µmol/L).5 A third chest x-ray was ordered, and the patient’s medication profile was reviewed multiple times for drugs interacting with MTX clearance that may have been missed or added. The patient’s urine pH, output, and renal function remained optimized despite clearance delay. At this time, the serum MTX level assay was reviewed for possible inaccuracy.
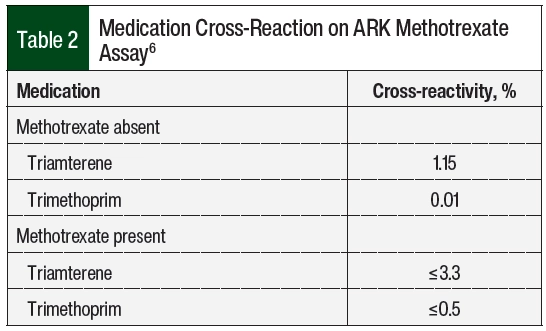
VA Boston Healthcare System sends its MTX serum levels to a large local medical center for processing. This hospital was contacted, and the case was discussed with the chemistry fellow. After review with the chemistry fellow, it was determined that the MTX assay was run using the ARK Diagnostics MTX assay.6 This homogeneous immunoassay cross-reacts slightly with triamterene and trimethoprim (Table 2).6 It was then identified that the hydrochlorothiazide plus triamterene that was administered to the patient may be causing a false serum MTX level to be present on the assay. After the discontinuation of hydrochlorothiazide plus triamterene, the subsequent serum MTX level demonstrated clearance with 0.04 µmol/L on day 11 and an undetectable level on day 12. Leucovorin was administered every 6 hours and was then discontinued on day 12.
Discussion
HD-MTX is a regimen that is used for multiple cancers across hematology and oncology. The risk for severe adverse events merits a high level of collaboration between clinical pharmacy, hematology/oncology service, and the primary medical and nursing teams in the hospital treatment unit. Special attention is given to any medications that interact with protein binding, renal transport, or reduced renal function because they can delay MTX elimination and increase adverse events.1,2 Although tertiary database interaction checkers are focused on the drug-drug interactions that are associated with actual MTX, there is minimal guidance on possible interactions in vitro with laboratory assays. The single case report of increased adverse events with triamterene plus MTX was a woman aged 57 years who received 5 mg weekly of MTX for the treatment of rheumatoid arthritis.7 The patient’s pretreatment and renal function were not noted and could not be ruled out as a cause of her adverse events. The researchers discussed that long-term treatment with triamterene could have rendered the patient folate-deficient, which could have contributed to the bone marrow adverse events she had.7 This mechanism of adverse events would not apply to our case report because intravenous leucovorin repletion was being administered per protocol.
A second case series linked a possible increase in myelosuppression in 14 patients with breast cancer who received treatment with cyclophosphamide, MTX, and 5-fluorouracil with concomitant thiazide diuretics.8 The MTX dose in this study was not high dose, and urine alkalinization and leucovorin rescue were not required. The nature of this interaction was not determined.8 It is unlikely that clearance of MTX would have been affected by the use of hydrochlorothiazide in a patient with optimized hydration, urine output, and urine pH.
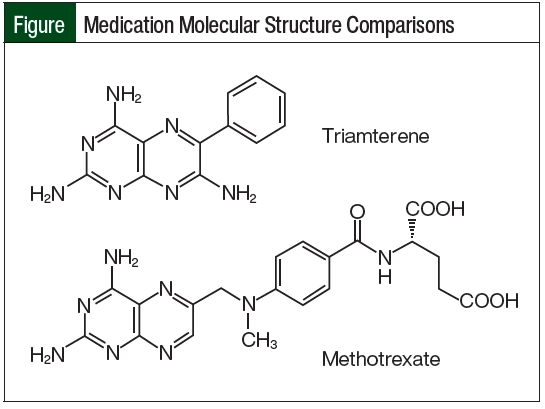
The most likely mechanism for delayed MTX elimination in our patient was related to assay cross-reactivity with triamterene. Triamterene’s molecular structure closely matches the pteridine ring moiety of MTX (Figure). Because of this similarity, triamterene can cross-react with the ARK immunoassay and show a false serum MTX level, even after a patient has entirely cleared the medication.6 This interaction was only identified after calling the laboratory conducting the assay for specific details about the test.
Triamterene has an elimination half-life of 1.5 to 2.5 hours, which can be prolonged to 6.5 hours in an elderly patient, and that may be modified by the coadministration of hydrochlorothiazide.9,10 A total of 18 hours elapsed between the last dose of hydrochlorothiazide 25 mg plus triamterene 37.5 mg and the next check of serum MTX, which returned at the lower limit of detection (0.04 µmol/L).7 One final MTX level 24 hours later showed full clearance. The reduction in ARK MTX assay detection with the discontinuation of triamterene would be a reasonable correlate with expected triamterene elimination in an elderly patient.
This case highlights the importance of evaluating the patient clinically instead of relying solely on laboratory results, as well as mindfulness that false-positives or false-negative results can result from the interaction of medications and laboratory reagents or assays. After an extensive literature search, this assay interaction has not been reported in any publications except the ARK MTX assay package insert.
Conclusion
As demonstrated by this case, there is an interaction between triamterene and the ARK MTX immunoassay because triamterene and MTX share a similar pteridine ring moiety. Healthcare providers who prescribe HD-MTX should be aware of this interaction because it can lead to falsely elevated MTX levels that lead to unnecessary supportive care, prolonged hospital stays, and more important, a delay in subsequent cycles of chemotherapy treatment.
Acknowledgments
The authors would like to acknowledge and thank the inpatient clinical pharmacy staff of the VA Boston Healthcare System, hematology/medical oncology fellow Britney Bell, MD, and pharmacy resident Christian Santiago, PharmD, RPh, for their discussion and involvement in the care of the patient presented.
Author Disclosure Statement
Dr Wood, Dr Brennan, Dr Wu, and Dr Hankins have no conflicts of interest to report.
References
- Methotrexate injection, for intravenous use [prescribing information]. Hospira, August 2022. Accessed March 18, 2024. https://labeling.pfizer.com/ShowLabeling.aspx?id=5380
- Howard SC, McCormick J, Pui CH, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): B-cell lymphomas. Version 1.2024. January 18, 2024. Accessed March 18, 2024. www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Central Nervous System Cancers. Version 1.2023. March 24, 2023. Accessed March 18, 2024. www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- Leucovorin calcium, for injection [prescribing information]. Hikma Pharmaceuticals USA, October 2021. Accessed March 18, 2024. www.dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=cc52077f-5663-4f79-a273-0aecf655e038&type
- ARK methotrexate assay [package insert]. ARK Diagnostics, November 2023. Accessed March 18, 2024. www//ark-tdm.com/products/cancer/methotrexate/pdfs/1600-0213-00_ARK_Methotrexate_Assay_Website_Rev10.pdf
- Richmond R, McRorie ER, Ogden DA, et al. Methotrexate and triamterene—a potentially fatal combination? Ann Rheum Dis. 1997;56:209-210.
- Orr LE. Potentiation of myelosuppression from cancer chemotherapy and thiazide diuretics. Drug Intell Clin Pharm. 1981;15:967-970.
- Pruitt AW, Winkel JS, Dayton PG. Variations in the fate of triamterene. Clin Pharmacol Ther. 1977;21:610-619.
- Mutschler E, Gilfrich HJ, Knauf H, et al. Pharmacokinetics of triamterene. Clin Exp Hypertens A. 1983;5:249-269.