Methotrexate (MTX) is an antimetabolite that reversibly binds dihydrofolate reductase, inhibiting the conversion of dihydrofolate to tetrahydrofolate and thus disrupting DNA synthesis, repair, and cellular replication.1 High-dose (HD) MTX (HD-MTX), most often defined as ≥1000 mg/m2, is employed for a variety of indications including acute lymphoblastic leukemia, lymphoma, and osteosarcoma.1-4 Without extensive monitoring and supportive care strategies, HD-MTX therapy is associated with a significant risk of adverse events that can lead to treatment delays, morbidity, and mortality.4
Numerous adverse events are associated with HD-MTX therapy. Although generally reversible, nephrotoxicity can occur in up to 12% of patients and is primarily caused by intratubular crystal formation, which occurs due to precipitation of MTX and its metabolites in acidic urine.4 At an acidic pH, MTX is poorly soluble and more likely to crystallize. Alkalinization increases MTX solubility; therefore, vigorous hydration with urinary alkalinization is recommended in all patients receiving HD-MTX therapy to prevent acute kidney injury.4 Mucositis and myelosuppression occur due to the cellular damage caused by the cytotoxic effects of MTX, and the risk of these adverse events increases with prolonged MTX exposure seen with delayed elimination.4 Transient liver toxicity may occur in up to 60% of patients, and the most common manifestations include elevated liver enzymes and hyperbilirubinemia.4 In addition, central nervous system (CNS) disturbances have been reported in up to 11% of patients receiving HD-MTX therapy.4 These disturbances may include encephalopathy and headache, and in severe cases, patients may have seizures.4
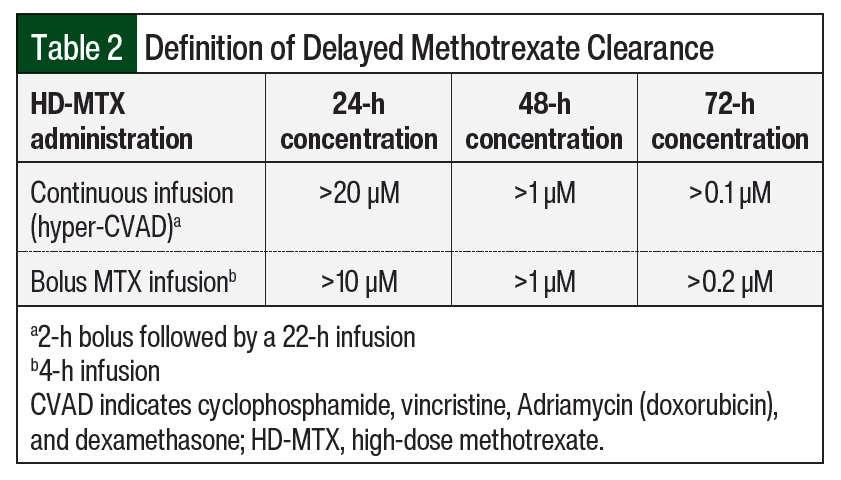
Supportive care strategies are recommended to prevent adverse events associated with delayed MTX elimination and include discontinuing interacting medications and optimizing leucovorin dosing.4 Multiple drug–drug interactions can contribute to delayed MTX clearance and enhance associated adverse events. Clinically significant drug interactions with MTX that are well documented in the literature include nonsteroidal anti-inflammatory drugs, penicillin and penicillin derivatives, salicylates, loop diuretics, sulfonamides, and proton pump inhibitors.4-7 Many HD-MTX protocols recommend the cessation of these medications during concurrent MTX therapy.4 Leucovorin acts as a “rescue” agent for MTX by providing normal cells with folates needed for DNA synthesis. It is typically initiated 24 to 36 hours after MTX infusion at 10 to 15 mg/m2 every 6 hours and continued until MTX clearance, defined as a level ≤0.1 µM in most protocols.1,4 Monitoring during HD-MTX therapy is essential as leucovorin rescue should be guided by serial serum MTX levels to protect against adverse events.4 In patients with evidence of delayed MTX clearance, which is often defined as MTX levels ≥10 µM at 24 hours, ≥1 µM at 48 hours, and ≥0.2 µM at 72 hours, leucovorin doses should be adjusted to prevent severe toxicities.8
Although protocols for leucovorin dose adjustments vary across institutions, standardizing HD-MTX monitoring has consistently led to improved patient outcomes.9,10 In February 2014, due to increased use of MTX-containing regimens, our institution implemented an HD-MTX management guideline. The document contains detailed information on MTX pharmacology and key adverse events, clinically significant drug interactions with MTX, and recommendations for supportive care measures, such as intensive hydration with urinary alkalinization and leucovorin (including dose adjustments). Although a valuable resource, a gap between guideline recommendations and clinical practice continued to exist and despite extensive physician and nursing education, adherence to the guideline remained low, resulting in inconsistent delivery of best supportive care to patients receiving HD-MTX. In an effort to standardize and improve existing processes, a pharmacist-driven HD-MTX protocol was implemented in August 2017 that enabled decentralized pharmacists to closely monitor patients receiving MTX using the guidelines developed in 2014.
The purpose of our study was to evaluate the impact of a pharmacist-driven HD-MTX monitoring protocol on improving the management of delayed MTX elimination and preventing MTX-associated adverse events.
Methods
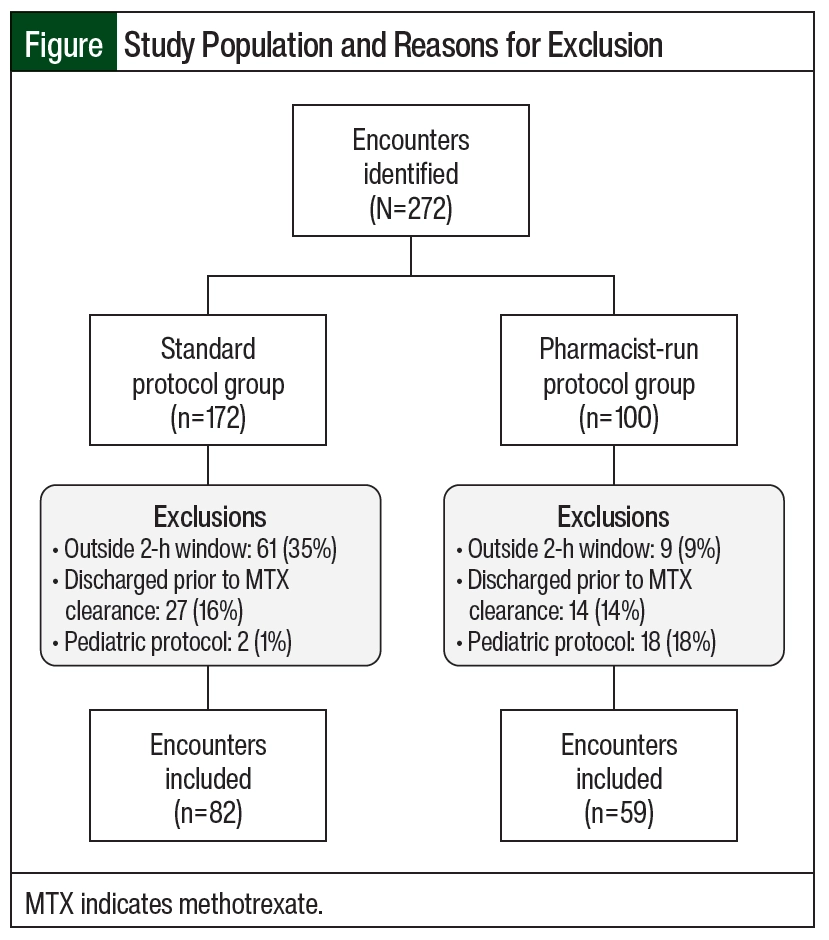
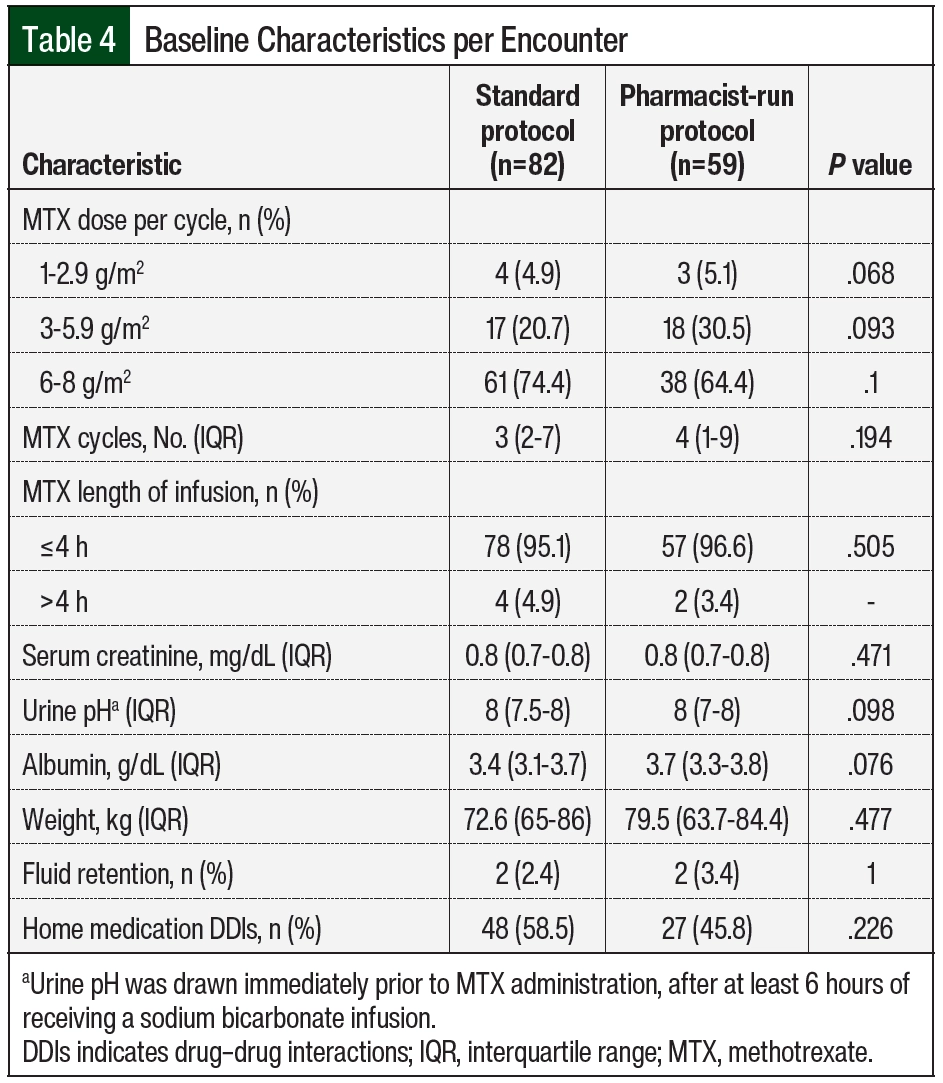
This was a single-center, retrospective chart review conducted at a 923-bed independent academic medical center with a dedicated 24-bed inpatient adult oncology unit and an associated outpatient cancer center. The institutional electronic health record (Epic) and Web Intelligence reports identified patients aged ≥18 years who received HD-MTX between August 2014 and August 2020. This study consisted of 2 groups: a standard protocol group from August 2014 through July 2017 and a pharmacist-run protocol group from August 2017 through August 2020. Patients could be included in our analysis more than once if they had a repeat but separate hospital encounter. Patients were excluded if they were discharged before MTX clearance (defined as ≤0.1 µM) or if they received HD-MTX as part of a pediatric protocol, as many of these protocols have unique nomograms. Patient encounters where MTX concentrations were drawn outside of a 2-hour window from scheduled time were excluded from our analysis. The only exception was when assessing the end point focused on timing of MTX levels. These encounters were included to determine if the pharmacy-driven protocol improved timing of MTX concentrations. Data collected included patient’s age, sex, cancer diagnosis, chemotherapy regimen, MTX dose and rate of infusion, serum creatinine, urine pH, relevant drug interactions, weight, and the presence of ascites, pleural effusion, and fluid retention (Figure).
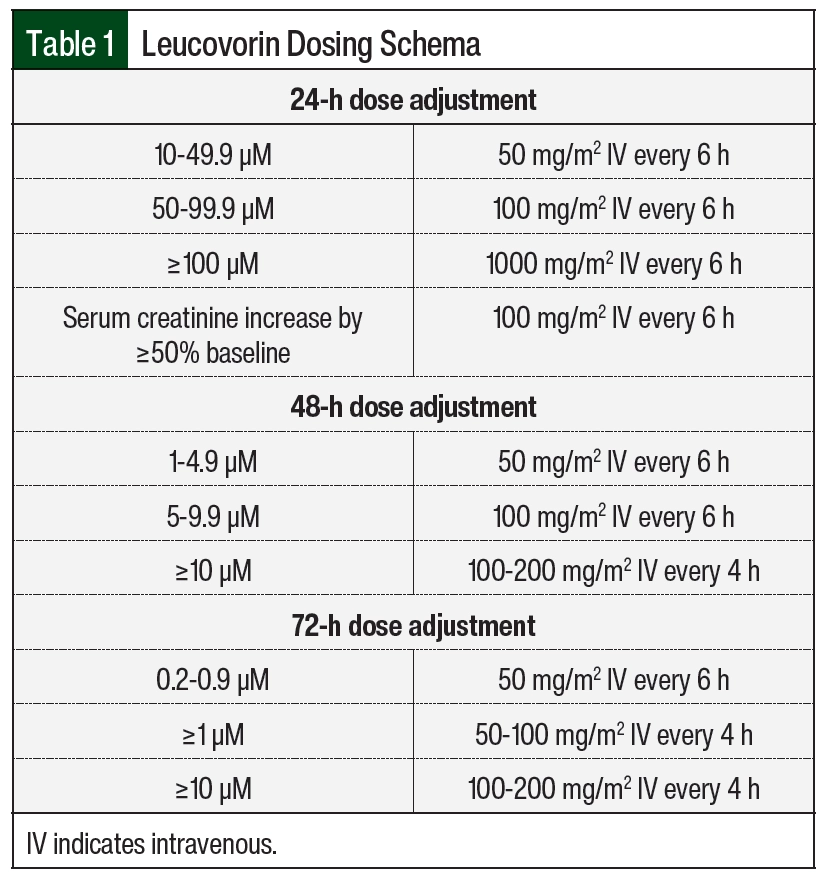
Patients at our institution treated with HD-MTX therapy received supportive care measures, including hydration/urinary alkalinization and leucovorin rescue. All patients received intravenous hydration with alkalinization starting 6 hours before the initiation of HD-MTX and continued until MTX clearance. Urine pH had to be ≥7 before starting the infusion and maintained at this level until leucovorin rescue was completed. Urine pH was monitored every 6 hours, and if urine pH was <7 at any time during therapy, a bolus of sodium bicarbonate 50 mEq (1 mEq/mL) over 15 minutes was administered. Patient weight was assessed daily, and strict ins and outs were recorded to monitor for changes in fluid status. Plasma MTX levels were drawn at 24, 48, and 72 hours after the start of the HD-MTX infusion and daily thereafter until concentrations were ≤0.1 µM. Serum creatinine and electrolytes were monitored on a daily basis to identify patients at highest risk for adverse events. Leucovorin was initiated at least 24 to 36 hours after the start of MTX infusion at 10 to 15 mg/m2 every 6 hours. The leucovorin dose was adjusted per our institution-specific protocol for patients with evidence of delayed clearance to prevent severe adverse events (Table 1 and Table 2).
The standardized pharmacist-run monitoring protocol allowed for daily MTX monitoring from 10:00 ᴀᴍ to 6:30 ᴘᴍ, including on weekends. Once a patient was admitted for HD-MTX therapy, before initiating treatment, pharmacists were responsible for reviewing the patient’s profile for drug–drug interactions, ensuring urine pH was monitored every 6 hours, and confirming that the patient’s serum creatinine and potassium levels, liver function tests, and weight were monitored daily. After the start of the infusion, the pharmacist was responsible for ordering MTX level monitoring and ensuring appropriate timing of levels relevant to initiation of MTX therapy; recommending leucovorin dosage adjustments in patients with supratherapeutic serum MTX concentrations at 24, 48, and 72 hours after the start of MTX infusion; and monitoring the patient’s daily weight, fluid balance, and laboratory parameters for additional supportive care measures or dose adjustments in subsequent cycles. A detailed pharmacy consult with recommendations was added to the patient’s chart to ensure timely transfer of information between healthcare providers.
The primary outcome was appropriateness of leucovorin dose adjustments, defined as leucovorin dose escalation according to our institution’s protocol for HD-MTX (Table 1). Secondary outcomes included time to MTX clearance measured from the start of infusion, defined as a concentration of ≤0.1 µM; MTX concentrations at 24, 48, and 72 hours; rate of delayed clearance; urine pH; length of stay; drug–drug interactions during HD-MTX therapy; accurate timing of MTX concentrations, defined as levels drawn within a 2-hour window of scheduled time; and incidence of adverse events as defined by the Common Terminology Criteria for Adverse Events version 5.0.11 Drug interactions assessed included sulfamethoxazole/trimethoprim, salicylates, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, penicillins, loop diuretics, and aminoglycosides.5-7,12,13 Baseline laboratory values, weight, fluid-retention status, and drug–drug interactions were included in our analysis if they were collected within 72 hours of HD-MTX administration.
Data were analyzed based on HD-MTX encounters via IBM SPSS version 27. Descriptive statistics were used to evaluate baseline characteristics. Continuous variables were analyzed using the Mann–Whitney U test, and categorical variables were analyzed using the chi-squared or Fisher’s exact test. A P value of <.05 was used to indicate significance.
Results
A total of 272 encounters were initially identified. After exclusion criteria were applied, a total of 141 encounters were included in the analysis. Of those, 82 encounters were included in the standard protocol group and 59 encounters were included in the pharmacist-run protocol group (Figure). Only 1 patient included in our analysis had encounters in both the standard protocol and the pharmacist-run protocol groups. In the standard protocol group, 35% of encounters were excluded because of inappropriately timed MTX levels. After protocol implementation, however, only 9% of encounters were excluded due to inappropriately timed MTX levels. The majority of encounters excluded from the pharmacist-run protocol group were due to patients receiving HD-MTX as part of a pediatric protocol. The Figure outlines the reasons for exclusions.
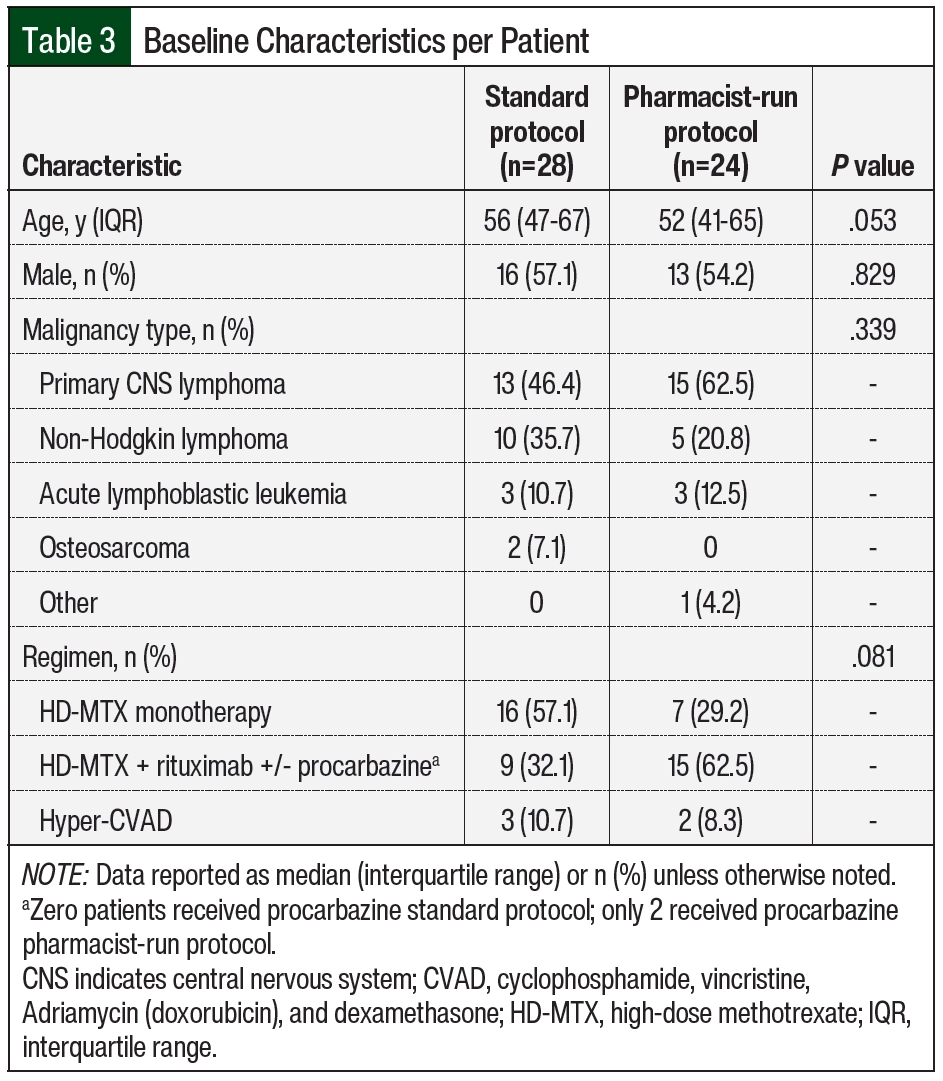
Baseline characteristics are summarized in Table 3 and Table 4. The median age of patients in the standard protocol group was 56 years, and 16 (57%) were men. The median age of patients in the pharmacist-run protocol group was 52 years, and 13 (54%) were men. The most common malignancy among both groups was primary CNS lymphoma followed by non-Hodgkin lymphoma. HD-MTX monotherapy was the most common regimen used in the standard protocol group (57.1%); whereas combination therapy with rituximab with or without procarbazine was the most common regimen used in the pharmacist-run protocol group (62.5%). More than 70% of encounters in the standard protocol group received 6 to 8 g/m2 of MTX compared with 64% in the pharmacist-run protocol group. The median number of HD-MTX cycles was 3 in the standard protocol group and 4 in the pharmacist-run protocol group. No significant differences were noted between baseline serum creatinine, urine pH, albumin, weight, rates of ascites, pleural effusion, or fluid retention and rates of interacting home medications between groups.
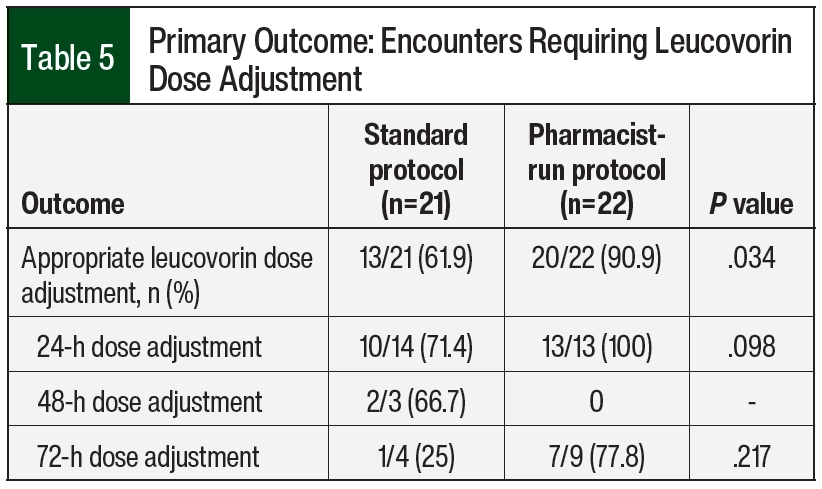
In all, 21 encounters in the standard protocol group and 22 encounters in the pharmacist-run protocol group required leucovorin dose escalations at 24, 48, or 72 hours (Table 5). The overall appropriateness of leucovorin dose adjustments improved after the implementation of the pharmacist-driven protocol (13 [61.9%] vs 20 [90.9%]; P=.034).
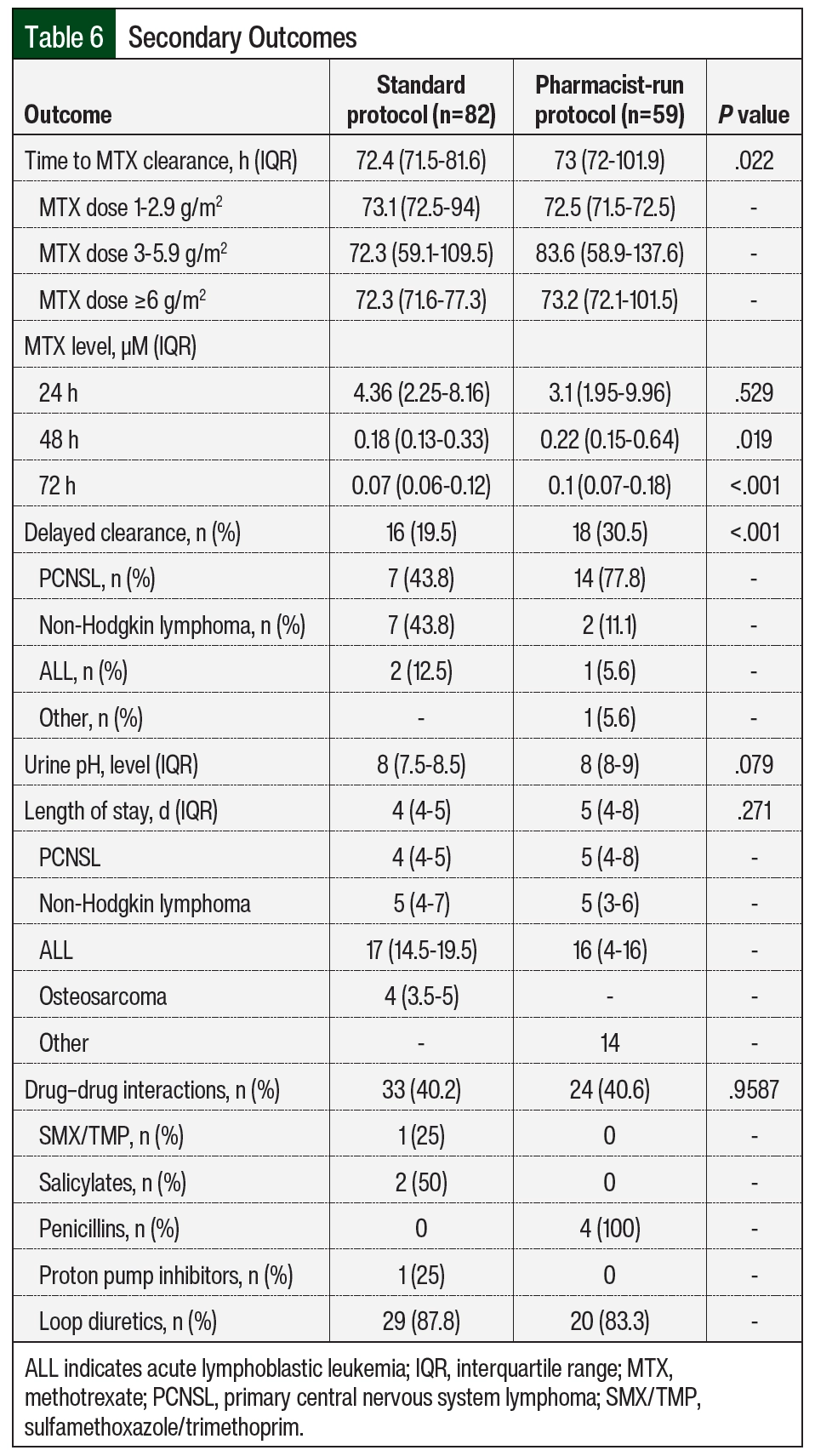
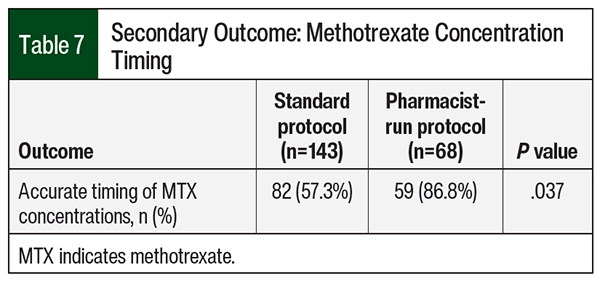
Although no significant difference was seen in MTX levels between the 2 groups at 24 hours, the median MTX concentration was higher in the pharmacist-run protocol group compared with the standard group at 48 and 72 hours—0.18 µM versus 0.22 µM (P=.019) and 0.07 µM versus 0.1 µM (P<.001), respectively (Table 6). Overall median time to MTX elimination was also slightly higher in the pharmacist-run protocol group: 73 hours versus 72.4 hours; P=.022. Although the differences are statistically significant, neither of these findings are clinically significant. Rates of delayed clearance were 19.5% (n=16) in the standard protocol group and 30.5% (n=18) in the pharmacist-run protocol group (P<.001). Delayed clearance in the pharmacist-run protocol group was documented mostly in patients with primary CNS lymphoma. Although not significant, median hospital length of stay was higher in the pharmacist-run protocol group. No significant differences were noted in urine pH or drug–drug interactions during MTX therapy between groups (Table 6). Accurate timing of MTX concentrations significantly improved pharmacist-run protocol (n=82 [57.3%] vs n=59 [86.8%]; P=.037; Table 7).
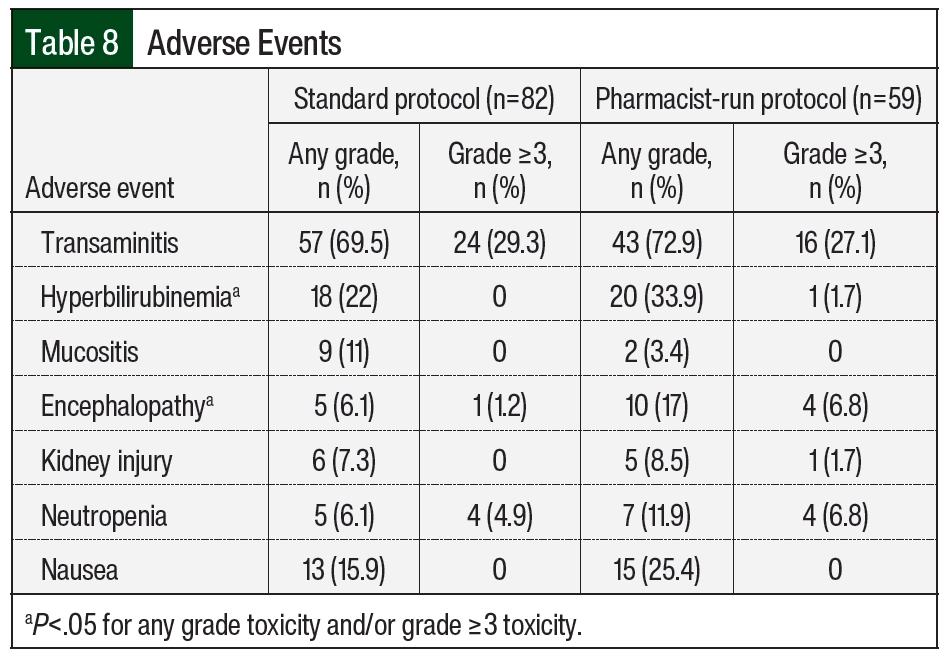
When assessing adverse events, the incidence of any-grade hyperbilirubinemia was higher in the pharmacist-run protocol group (n=18 [22%] vs n=20 [33.9%]; P=.019, respectively); the incidence of encephalopathy was also higher in the pharmacist-run protocol group (n=5 [6.1%] vs n=10 [17%]; P=.043, respectively). No differences in incidences of any other adverse events were noted between groups (Table 8).
Discussion
Although previous studies have demonstrated the benefit of standardized HD-MTX on clinical outcomes, there is a paucity of literature surrounding pharmacists’ impact on adherence to an HD-MTX protocol. A retrospective chart review by Cerminara and colleagues was conducted between 2009 and 2014 to evaluate whether the implementation of a standardized HD-MTX protocol improved adherence to consensus recommendations for the administration of HD-MTX therapy.9 The University of Maryland’s protocol was not solely pharmacist-driven, but instead involved the entire care team. Recommendations were developed regarding dosing information, supportive care, and drug–drug interactions for the physician to review when entering the orders. The clinical pharmacist reviewed the orders and after the MTX was initiated, made adjustments to ensure proper timing of monitoring of MTX levels and administering leucovorin doses. Escalation of leucovorin doses was conducted as part of a nursing-driven procedure. The study found that protocol implementation significantly reduced the deviation of monitoring MTX-level timing at 24, 48, and 72 hours and improved the time to initiation of leucovorin rescue.9
After this study, Matta and colleagues conducted a prospective cohort and retrospective chart review (2016 through 2019) to evaluate the impact of a pharmacist-driven HD-MTX protocol on inpatient length of stay and patient safety.10 The protocol required oncology pharmacists to screen for drug interactions, manage urinary alkalinization, monitor renal function and serum MTX levels, and initiate and adjust leucovorin orders.10 This study demonstrated decreases in overall length of stay and the incidence of adverse events (acute kidney injury, anemia, neutropenia) after protocol implementation; however, its results are limited due to the small number of charts available for review and heterogeneity of the population.10 These studies provide insight into pharmacists’ role in ensuring that appropriate supportive care strategies are initiated and continued throughout HD-MTX administration. Our study was conducted to contribute to the current literature and, to our knowledge, uses the largest sample size to evaluate HD-MTX monitoring protocols. Our study demonstrated the positive impact pharmacists have on ensuring patients receive optimal monitoring of HD-MTX to help prevent adverse events.
We observed an increased rate of delayed MTX clearance in the pharmacist-run protocol group (Table 6), which may be explained by the baseline demographics of this group (Table 3). Although not significant, the median number of HD-MTX cycles was greater in the pharmacist-run protocol group. It has been described in the literature that cumulative MTX exposure (>4-5 cycles) is a risk factor for delayed elimination.14 We also observed increased adverse events in the pharmacist-run protocol group, which is most likely secondary to the fact that many pharmacist-run protocol patients received HD-MTX in combination with other agents; whereas patients in the standard protocol group mostly received MTX as monotherapy. In addition, primary CNS lymphoma was seen at a higher prevalence in the pharmacist-run protocol group, which may have contributed to the increased encephalopathy noted in this population.15
Although leucovorin rescue is a universal recommendation after HD-MTX administration, a retrospective single-center study conducted by Barreto and colleagues found no utility in empirically escalating doses in subsequent HD-MTX cycles after a patient had previously demonstrated delayed elimination.16 This study parallels our own in that a reduction in delayed HD-MTX elimination was not demonstrated despite appropriate leucovorin escalations.16 These results indicate that for patients at high risk of delayed elimination, leucovorin dose escalations alone may not be sufficient, and further mechanisms of risk mitigation should be explored. As Barreto and colleagues discuss, individualizing the HD-MTX to provide both clinical effectiveness and safety is crucial, and the use of novel renal biomarkers may improve dose optimization.16 As these technologies are explored and advances to improve patient care occur, pharmacist involvement is critical.
This study has several limitations. First, due to the retrospective nature of the study, data collection was dependent on accurate documentation. Second, due to the expected limited number of patients and the heterogeneity of patients being treated with HD-MTX, our primary outcome and most secondary outcomes were measured using surrogate end points. In addition, many of the patients were receiving MTX in combination with other cytotoxic agents, which caused difficulties in attributing all adverse events to MTX therapy. Furthermore, we were unable to follow our patients after discharge, so delayed adverse events, such as neutropenia, may have been incompletely captured in our data. Conversely, no time frame was specified for attributing adverse events to HD-MTX during patient hospitalizations, creating the risk for recording falsely higher incidence of adverse events after HD-MTX if a patient experienced an extended stay. Although the decentralized oncology pharmacists perform HD-MTX monitoring every day, they are only available from 10:00 ᴀᴍ to 6:30 ᴘᴍ, which may cause delays in dose escalation if the MTX concentration results after hours. Similarly, excluding encounters where MTX concentrations were drawn outside of a 2-hour window likely decreased this study’s external validity, as many centers may not be capable of adhering to this strict time constraint. Because these patients were excluded and the percentage of patients with levels drawn appropriately was much greater with the pharmacist-run protocol, the actual difference in appropriate leucovorin dose adjustments may be larger than reported. Finally, the protocol was based on institutional guidelines implemented in 2014. Because of this, the current difference in outcomes may be diminished due to some adherence to the guidelines before protocol implementation in August 2017.
Conclusion
The implementation of a pharmacist-driven HD-MTX monitoring protocol resulted in a significant improvement in the rate of appropriate leucovorin dose escalations. This study demonstrates the value that pharmacists add to monitoring a high-risk regimen and ensuring that proper management occurs. Although the primary objective of this study was met, an imbalance in patient characteristics likely led to our inability to show a significant impact on clinical outcomes. Given the lack of prospective literature, as this study and others are retrospective, further research is needed to confirm the impact of a pharmacist-driven HD-MTX protocol on clinical outcomes.
References
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694-703.
- Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547-561.
- Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21:1044-1049.
- Howard SC, McCormick J, Pui CH, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482.
- Levêque D, Santucci R, Gourieux B, Herbrecht R. Pharmacokinetic drug-drug interactions with methotrexate in oncology. Expert Rev Clin Pharmacol. 2011;4:743-750.
- Santucci R, Levêque D, Lescoute A, et al. Delayed elimination of methotrexate associated with co-administration of proton pump inhibitors. Anticancer Res. 2010;30:3807-3810.
- Wiczer T, Dotson E, Tuten A, et al. Evaluation of incidence and risk factors for high-dose methotrexate-induced nephrotoxicity. J Oncol Pharm Pract. 2016;22:430-436.
- Leucovorin calcium [prescribing information]. Bedford Laboratories; November 2011.
- Cerminara Z, Duffy A, Nishioka J, et al. A single center retrospective analysis of a protocol for high-dose methotrexate and leucovorin rescue administration. J Oncol Pharm Pract. 2019;25:76-84.
- Matta SF, Gieselman LA, Mancini RS. A single-center qualitative study evaluating the safety and efficacy of a pharmacist-driven protocol for high-dose methotrexate management. J Oncol Pharm Pract. 2021;27:588-595.
- National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. November 27, 2017. Accessed May 17, 2021. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/doc/CTCAE_v5_Quick_Reference_5x7.pdf
- de Miguel D, García-Suárez J, Martín Y, et al. Severe acute renal failure following high-dose methotrexate therapy in adults with haematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23:3762-3766.
- Bezabeh S, Mackey AC, Kluetz P, et al. Accumulating evidence for a drug-drug interaction between methotrexate and proton pump inhibitors. Oncologist. 2012;17:550-554.
- Green MR, Chamberlain MC. Renal dysfunction during and after high-dose methotrexate. Cancer Chemother Pharmacol. 2009;63:599-604.
- Bhagavathi S, Wilson J. Primary central nervous system lymphoma. Arch Pathol Lab Med. 2008;132:1830-1834.
- Barreto JN, Peterson KT, Barreto EF, et al. Early, empiric high-dose leucovorin rescue in lymphoma patients treated with sequential doses of high-dose methotrexate. Support Care Cancer. 2021;29:5293-5301.