Symptom Overview
A paradigm shift in the management of relapsed or refractory B-cell chronic lymphocytic leukemia (CLL) and low-grade non-Hodgkin lymphoma (NHL) has evolved in recent years.1 With increased use of targeted therapies, ibrutinib, an oral Bruton’s tyrosine kinase (BTK) inhibitor, is an integral therapeutic option for patients with treatment-naïve and treatment-experienced CLL or low-grade NHL.2 Ibrutinib therapy demonstrated superior overall response rates compared with ofatumumab and other agents in treatment-experienced patients with CLL.3,4
Etiology
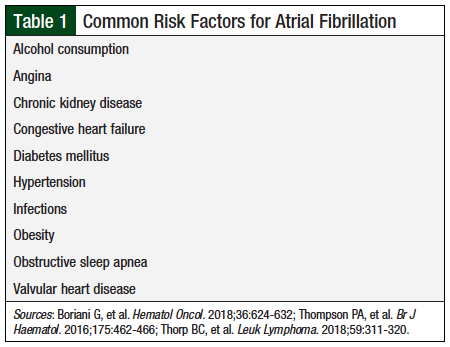
The approved treatment duration with ibrutinib is until disease progression or unacceptable toxicity.2 However, long-term treatment with ibrutinib is associated with increased rates of atrial fibrillation (AF),2 which may be a class effect of BTK inhibitors, because it also occurs with acalabrutinib treatment.5 The incidence of AF reported in clinical trials with ibrutinib varies between 6% and 9%6,7 and depends on patients’ risk factors (Table 1).6-8
The treatment of ibrutinib-related AF requires a concomitant use of anticoagulants based on patients’ risk factors for stroke.6 Anticoagulant selection is complicated by an increased risk for systemic bleeding (up to 50%) with ibrutinib, as was shown in clinical trials.6,7 Currently, no systemized guidance exists regarding the appropriate anticoagulant choice for the management of ibrutinib-related AF.
Anticoagulation Management
The decision to start anticoagulation in patients with AF is based on the assessment of risks versus benefits and is done with specific risk-stratification tools—the HAS-BLED scoring system for bleeding risk and the CHA2DS2-VASc scoring system for thrombosis risk.6,9 These tools have also been proposed for use in deciding the appropriate approach to ibrutinib-related AF anticoagulation.6,9
However, the clinical benefit of these tools is questionable for patients with ibrutinib-related AF. These tools have not been validated in patients with hematologic malignancies, such as CLL or NHL. Therefore, their use may not accurately reflect the bleeding or thrombosis risks for patients with hematologic tumors. In addition, ibrutinib’s antiplatelet actions confound the bleeding risk assessment for patients with ibrutinib-related AF.
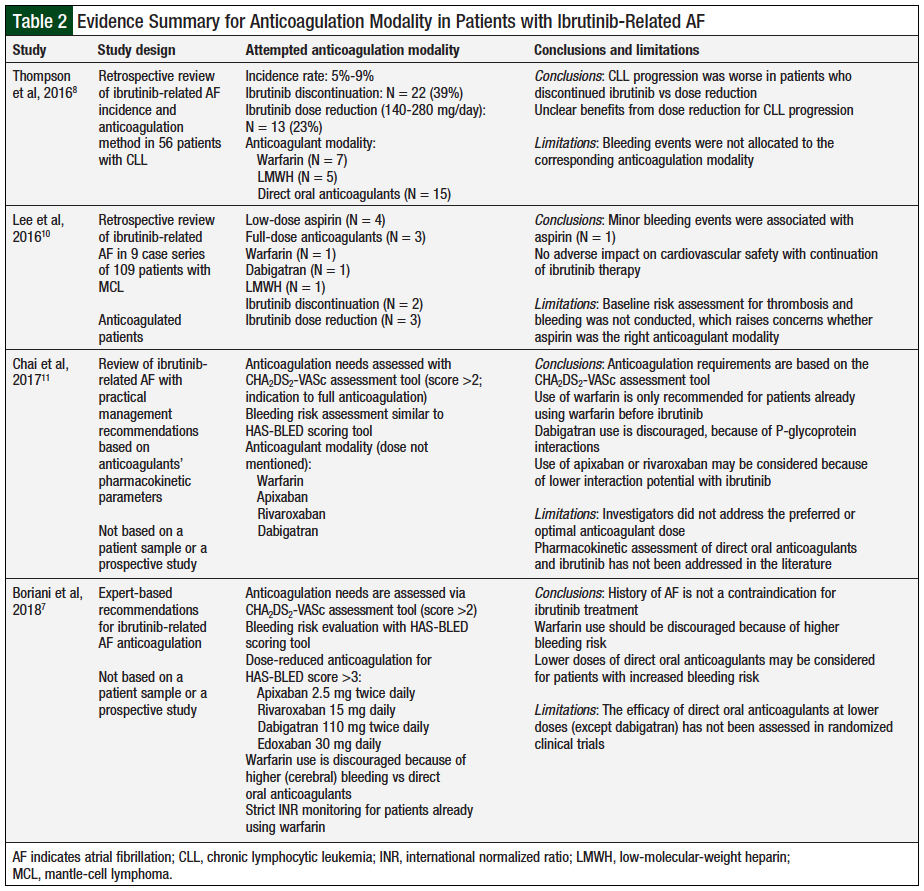
The authors of a comprehensive review of ibrutinib-related AF attempted to create an algorithm to manage ibrutinib-related AF.9 The researchers included 9 studies encompassing 1772 patients with lymphocytic leukemia or lymphoma and evaluated their bleeding events, concomitant use of anticoagulants, and associated adjustments in therapy, as well as AF events and associated adjustments in therapy. The researchers highlighted the challenges in the management of ibrutinib-related AF, emphasizing that the need for anticoagulation may be hindered by the increased risk for hemorrhage from ibrutinib use.9 Furthermore, they noted that applying the HAS-BLED scoring system may underestimate the bleeding risk for these patients, because it does not account for the increased bleeding risk underlined by hematologic malignancies.9 Multiple publications addressing anticoagulation for ibrutinib-related AF management are outlined in Table 2.7,8,10,11
A retrospective study included 56 patients with CLL who had received ibrutinib therapy and had ibrutinib-related AF.8 Overall, 51 patients received ibrutinib monotherapy and 5 patients received ibrutinib plus rituximab.8 In all, 73% of the patients received thromboprophylaxis with antiplatelet agents and/or with anticoagulants. In addition, 48% of patients received therapeutic anticoagulation—7 received warfarin, 5 received low-molecular-weight heparin (LMWH)—and 15 patients received direct oral anticoagulants (ie, dabigatran, apixaban, or rivaroxaban).8 Grade 3 to 4 bleeding (2 gastrointestinal [GI], 2 hemorrhagic cardiac tamponade, 1 intrapulmonary hemorrhage, 3 hematomas) was observed in 8 (14%) patients. Furthermore, 22 of the 56 patients permanently discontinued ibrutinib therapy. Of the 34 patients who continued therapy, 21 continued therapy at a full dose of ibrutinib.8
During the study, 14 of the 56 patients had disease progression; of these, 4 of 21 (19%) patients received 420 mg daily of ibrutinib, 2 of 13 (16%) received 140 mg to 280 mg daily, and 8 of 22 discontinued treatment.8 The investigators concluded that the study was not large enough to provide specific ibrutinib-related AF management recommendations, and that the disease progression outcomes after ibrutinib discontinuation were poor compared with dose reduction.8
Another retrospective review included 105 patients with relapsed mantle-cell lymphoma who received ibrutinib treatment with or without rituximab.10 New-onset AF occurred in 9 patients, of whom 4 received aspirin only, and 3 patients received full anticoagulation (ie, LMWH or dabigatran). Aspirin and anticoagulant doses were not defined in the review. The authors concluded that aspirin was a safe and effective treatment for ibrutinib-related AF, with 3 patients having nonfatal GI bleeding. Thromboembolic outcomes were comparable with dose reduction versus continuing ibrutinib therapy at the same dose.10
In the absence of baseline stroke risk assessment (using the CHA2DS2-VASc system), aspirin efficacy as an anticoagulant for ibrutinib-related AF is still undetermined. In patients with high stroke risk at baseline, aspirin monotherapy may be insufficient for ischemic stroke prevention.10 Given the study design, and the relatively small number of patients with ibrutinib-related AF,10 the researchers’ conclusion must be evaluated with a more robust design, defined drug doses, and an appropriate follow-up period for thromboembolic complications versus systemic bleeding.
A recent review of anticoagulant choices for ibrutinib-related AF thromboprophylaxis stated that apixaban and rivaroxaban are 2 appropriate options for concomitant use with ibrutinib.11 The researchers’ recommendations are based on a pharmacokinetic rationale that despite being cytochrome (CY)P3A4 substrates, the concomitant use of rivaroxaban and apixaban with ibrutinib may lead to a clinically nonsignificant increase in apixaban or rivaroxaban serum levels.11
Dabigatran 150 mg should be discouraged for use with ibrutinib, because it is a potent P-glycoprotein substrate and ibrutinib is a P-glycoprotein inhibitor.7 A recent article by a team of experts on ibrutinib-related AF management discouraged the use of warfarin, because of an increased risk for cerebral bleeding compared with direct oral anticoagulants.7 This may be a significant issue with ibrutinib, based on a case report of ibrutinib-induced cerebral aneurysms.12 The expert team recommended low starting doses of direct oral anticoagulants (ie, apixaban 2.5 mg; rivaroxaban 15 mg; edoxaban 30 mg; and dabigatran 110 mg) for 7 days, titrated to the maintenance doses.7
The efficacy data for subtherapeutic direct oral anticoagulant dosing in patients with B-cell lymphoma or leukemia are scarce. An initial low dose of direct oral anticoagulants (ie, first 7-10 days), followed by approved maintenance doses based on patients’ tolerability, is a potential strategy; however, this strategy has not been tested to account for increased bleeding risks in patients with CLL or with lymphoma while using ibrutinib therapy at full therapeutic doses.
Other approaches for ibrutinib-related AF management include dose reduction (to 140 mg/day) or to brief discontinuation of ibrutinib6-9; however, the impact of either approach on the cancer progression should be evaluated in large, controlled clinical trials.
Ibrutinib’s manufacturer currently states that the drug may be interrupted for any grade 3 or greater nonhematologic toxicities, including ibrutinib-related AF.2 Once the associated toxicity has reverted to grade 1 or to baseline (ie, recovery), therapy may be reinitiated at the previous dose. If the toxicity recurs, the manufacturer recommends dose reduction by 1 capsule (140 mg/day).2 However, this approach is largely driven by the exclusion of concomitant anticoagulant use in the pivotal clinical trials.2
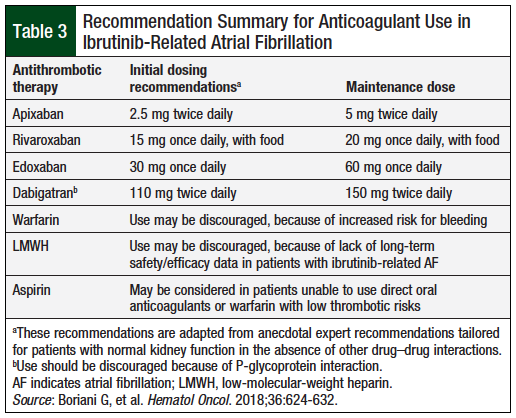
Table 3 provides anticoagulant dosage recommendations when anticoagulation is warranted for the management of ibrutinib-related AF.
Conclusion
Ibrutinib-related AF management with anticoagulants is challenging because of increased bleeding risks secondary to ibrutinib’s antiplatelet effect. Data regarding optimal anticoagulant selection in this scenario are limited to small, retrospective studies. Of the available therapies in the United States, oral factor Xa inhibitors (ie, apixaban, rivaroxaban, and edoxaban) are potential options, based on clinically insignificant CYP3A4 interactions with ibrutinib. Oral Xa inhibitors may be started at a reduced dose for the initial 7 to 10 days, followed by titration to maintenance doses based on patients’ tolerance. Warfarin may also be considered; however, because of an increased risk for bleeding (especially cerebral bleeding) with concomitant ibrutinib use, warfarin may be reserved for patients who have received warfarin therapy before initiation of ibrutinib therapy and are unable to take oral Xa inhibitors.
Frequent monitoring of international normalized ratio is recommended for patients taking warfarin with ibrutinib. Because dabigatran is a major substrate for P-glycoprotein, its use with ibrutinib is discouraged. Other therapeutic options include ibrutinib discontinuation or dose reduction. However, the impact of long-term dose reductions on malignancy progression is unknown at present.
Author Disclosure Statement
Dr Bubalo received honoraria from Janssen Pharmaceuticals. Dr Al-Jammali and Dr Beckner have no conflicts of interest to report.
References
- Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92:946-965.
- Imbruvica (ibrutinib) capsules/tablets [prescribing information]. Sunnyvale, CA: Pharmacyclics; Horsham, PA: Janssen Biotech; January 2019.
- Byrd JC, Brown JR, O’Brien S, et al; for the RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213-223.
- Chanan-Khan A, Cramer P, Demirkan F, et al; for the HELIOS Investigators. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200-211.
- Calquence (acalbrutinib) capsules, for oral use [prescribing information]. Willmington, DE: AstraZeneca Pharmaceuticals; November 2017.
- Thorp BC, Badoux X. Atrial fibrillation as a complication of ibrutinib therapy: clinical features and challenges of management. Leuk Lymphoma. 2018;59:311-320.
- Boriani G, Corradini P, Cuneo A, et al. Practical management of ibrutinib in the real life: focus on atrial fibrillation and bleeding. Hematol Oncol. 2018;36:624-632.
- Thompson PA, Lévy V, Tam CS, et al; on behalf of the FILO group. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175:462-466.
- Vrontikis A, Carey J, Gilreath JA, et al. Proposed algorithm for managing ibrutinib-related atrial fibrillation. Oncology (Williston Park). 2016;30:970-981, C3.
- Lee HJ, Chihara D, Wang M, et al. Ibrutinib-related atrial fibrillation in patients with mantle cell lymphoma. Leuk Lymphoma. 2016;57:2914-2916.
- Chai KL, Rowan G, Seymour JF, et al. Practical recommendations for the choice of anticoagulants in the management of patients with atrial fibrillation on ibrutinib. Leuk Lymphoma. 2017;58:2811-2814.
- Wiedower E, Hare F, Arthur A, et al. Unusual, spontaneous aneurysm formation in a patient being treated with ibrutinib for chronic lymphocytic leukemia. Ther Adv Hematol. 2016;7:231-232.