Cancer cells can escape the immune system by suppressing the T-cell–mediated immune response and creating an immunosuppressive microenvironment.1 The immunosuppressive tumor microenvironment exhibits elevated expression levels of co-inhibitory protein ligands, including the PD-L1 that, when bound to its receptor PD-1, can prevent T-cell–mediated immune response.1 Therefore, checkpoint inhibitor immunotherapy is aimed at overcoming tumor-mediated immune evasion by activating cytolytic T-cell responses in the tumor microenvironment.1 Pembrolizumab and nivolumab are PD-1–blocking antibodies that are FDA-approved for a variety of malignancies.2,3 Both of these antibodies block PD-L1–PD-1 interactions, resulting in the activation of T-cell–mediated immune responses.2,3 Based on initial studies, nivolumab was approved at a dose of 3 mg/kg intravenously (IV) every 2 weeks, and pembrolizumab was approved at a dose of 2 mg/kg IV every 3 weeks.4,5 However, fixed-dose schedules for pembrolizumab and nivolumab were evaluated because of the wide therapeutic index for checkpoint inhibitors to reduce the potential of dosing errors and to facilitate the production of these agents.6 As a result, nivolumab 3 mg/kg IV every 2 weeks was replaced with nivolumab 240 mg IV every 2 weeks for many indications.2 Similarly, pembrolizumab 2 mg/kg IV every 3 weeks was replaced with pembrolizumab 200 mg IV every 3 weeks for many indications.3
Along these same lines, an additional alternative fixed dosing schedule of nivolumab 480 mg IV every 4 weeks was approved by the FDA for nivolumab in 2018 based on modeling and simulation that predicted a similar benefit-risk profile for nivolumab 480 mg IV every 4 weeks compared with nivolumab 3 mg/kg IV every 2 weeks.7,8 In April 2020, the FDA granted accelerated approval for an additional new dosing regimen for pembrolizumab of 400 mg IV every 6 weeks.9,10 This approval was based on population pharmacokinetic analyses and exposure-response analyses that compared the predicted exposure of these agents to previously approved flat-dose regimens.7,10-12
Considering the mechanism of action of immune checkpoint inhibitors, which elicit a positive antitumor response through the activation and proliferation of T cells, the activated immune system can also attack normal body cells and tissues leading to the immune-related adverse events (irAEs).13 These irAEs can involve any organ system and most frequently include colitis, hepatitis, endocrinopathies, nephritis, and pneumonitis.13 Most irAEs are mild or moderate in severity and can be managed by holding immunotherapy agents and initiating supportive-care treatments until the resolution of symptoms to grade ≤1; however, some irAEs can be severe and potentially fatal, including pneumonitis, myocarditis, and myasthenia gravis.13 The National Comprehensive Cancer Network and the European Society for Medical Oncology have published guidelines on the management of adverse events related to immunotherapy.14,15
The incidence and prevalence of irAEs are still being evaluated in the literature, especially for the newer immunotherapy agents. In a pooled meta-analysis of 36 head-to-head, phase 2 and phase 3, randomized, controlled trials, the incidence of any-grade irAEs associated with a single agent varied widely across the different trials and agents, ranging from 15% to nearly 90% with atezolizumab showing the best safety profile, followed by nivolumab, pembrolizumab, ipilimumab, and tremelimimab.16 In addition to prevalence and incidence, another important consideration is the pattern of the time to onset and resolution of irAEs.13 A 2021 pooled analysis that included 22 studies involving 23 clinical trials and 8436 patients showed that the pooled median time to the onset of all-grade irAEs ranged from 2.2 weeks to 14.8 weeks, with renal events having the longest time to onset.13 The pooled median time to the resolution of all-grade irAEs ranged from 0.1 weeks to 54.3 weeks, with the shortest time in hypersensitivity and infusion reactions and the longest time in endocrine events.13 The 4 irAEs with the shortest time to onset were skin-related adverse events, hypersensitivity/infusion reactions, gastrointestinal events, and neurologic events.13 Although all-grade irAEs generally occurred within 14.8 weeks after the first dose of receiving an immunotherapy agent, severe irAEs were more likely to occur later than this time frame.13
The benefit-risk profile of an alternate dosing regimen for each agent is based on a relatively small sample size11 and mainly pharmacokinetic modeling studies6,7,10,12; therefore, additional studies evaluating their risk is worthwhile. The aim of this retrospective, single-center chart review was to compare the incidence of irAEs and infusion reactions between the different FDA-approved dosing regimens of nivolumab (nivolumab 240 mg IV every 2 weeks vs nivolumab 480 mg IV every 4 weeks) and pembrolizumab (pembrolizumab 200 mg IV every 3 weeks vs pembrolizumab 400 mg IV every 6 weeks).
The primary objective of this study was to assess the impact of alternate dosing schedules of pembrolizumab and nivolumab on the incidence of irAEs in the first 6 months after treatment initiation. The secondary objectives were to assess the impact of alternate dosing schedules for nivolumab and pembrolizumab on the incidence of infusion reactions, and the incidence of irAEs that led to a dose being held, the discontinuation of treatment, or hospitalization within 6 months of treatment initiation.
Methods
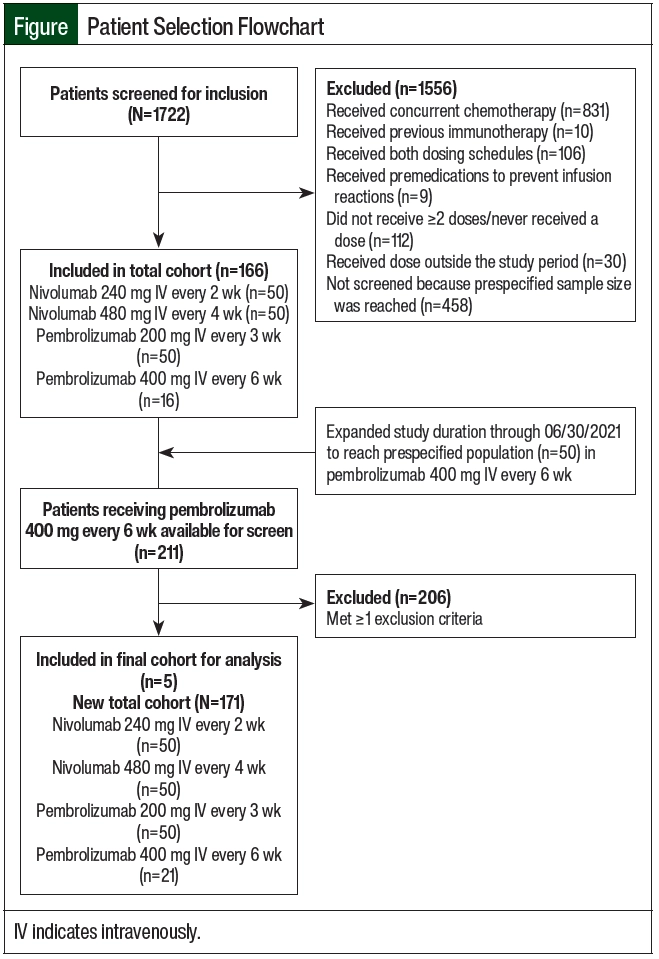
This single-center, retrospective cohort study was conducted at Duke University Health System (DUHS), a large tertiary and quaternary care academic medical center. Initially, the study period was January 1, 2018, through February 28, 2021, with a predefined sample population of 200 total patients who were equally divided among the 4 study arms of nivolumab 240 mg IV every 2 weeks, nivolumab 480 mg IV every 4 weeks, pembrolizumab 200 mg IV every 3 weeks, and pembroliz umab 400 mg IV every 6 weeks. All study arms reached the prespecified study population of 50 patients with any cancer diagnosis, except for the group that received pembrolizumab 400 mg IV every 6 weeks. Therefore, the study period was extended through June 30, 2021, to accommodate more patients to be included in the group that received pembrolizumab 400 mg IV every 6 weeks.
The patients who were initiated on treatment with nivolumab or pembrolizumab during the study period were identified using the reporting functionality within Epic, the electronic health record (EHR) used at DUHS. The study patients were aged ≥18 years and received at least 2 doses of one of the immunotherapy agents of nivolumab 240 mg IV every 2 weeks, nivolumab 480 mg IV every 4 weeks, pembrolizumab 200 mg IV every 3 weeks, or pembrolizumab 400 mg IV every 6 weeks within the first 6 months of treatment initiation for any cancer diagnosis. An additional study criterion was receiving treatment at Duke University Hospital, Duke Raleigh Hospital, or Duke Regional Hospital. Patients who were receiving concurrent chemotherapy; received an immunotherapy agent, including nivolumab, pembrolizumab, ipilimumab, durvalumab, avelumab, atezolizumab, or cemiplimab; received an immunotherapy agent at both dosing schedules; or received premedication to prevent infusion reactions before their first treatment were excluded from the study (Figure). This study was approved by the DUHS Institutional Review Board with a waiver of informed consent.
The primary study outcome was the incidence of any-grade irAEs that occurred and were associated with alternate dosing schedules of nivolumab and pembrolizumab within 6 months of treatment initiation. The secondary outcomes were the incidences of irAEs that required a dose to be held, required treatment discontinuation, required hospitalization, resulted in any-grade infusion reactions, or resulted in grade 3 and 4 infusion reactions with alternate dosing schedules of nivolumab and pembrolizumab within 6 months of treatment initiation.
The included patients’ data points were extracted from the EHR and were entered into the Research Electronic Data Capture electronic database. The baseline demographics, such as race, age, sex, type of malignancy, stage of malignancy, pre-existing comorbid conditions (including hypothyroidism, hyperthyroidism, chronic kidney disease, and liver failure), and the type of immunotherapy, dose of immunotherapy, infusion reactions, and irAEs were collected.
Statistical Analysis
Descriptive statistics were performed to summarize the patients’ demographics and clinical variables. Continuous variables were summarized using mean, median, standard deviation, 25th and 75th percentiles, and range. Categorical variables were summarized with frequency counts and percentages of nonmissing values. For the primary outcome, a chi-square test was used to study the association between the dose schedule and the incidence of irAEs. All statistical tests were 2-sided and were assessed at a significance level of 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC).
Results
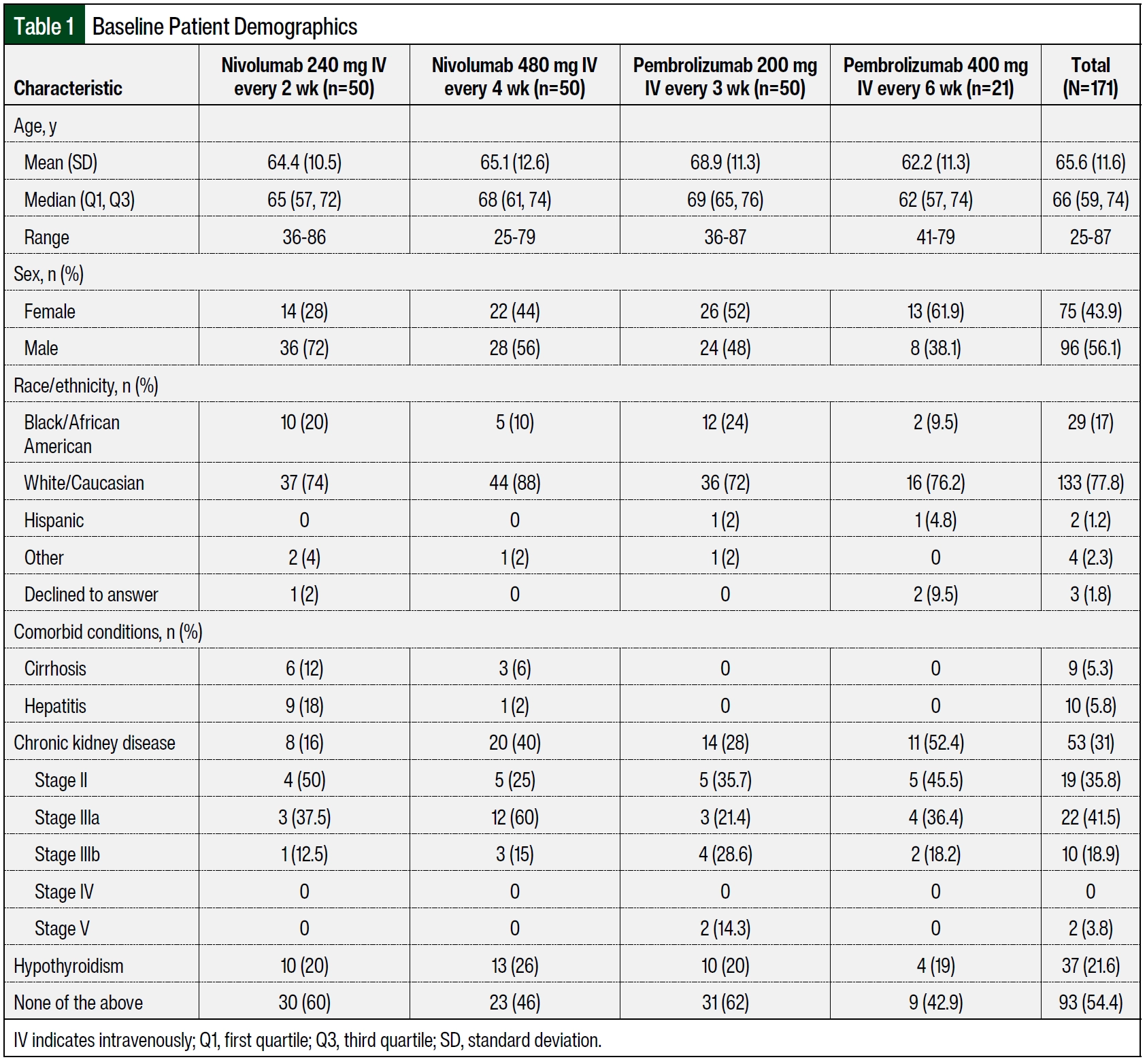
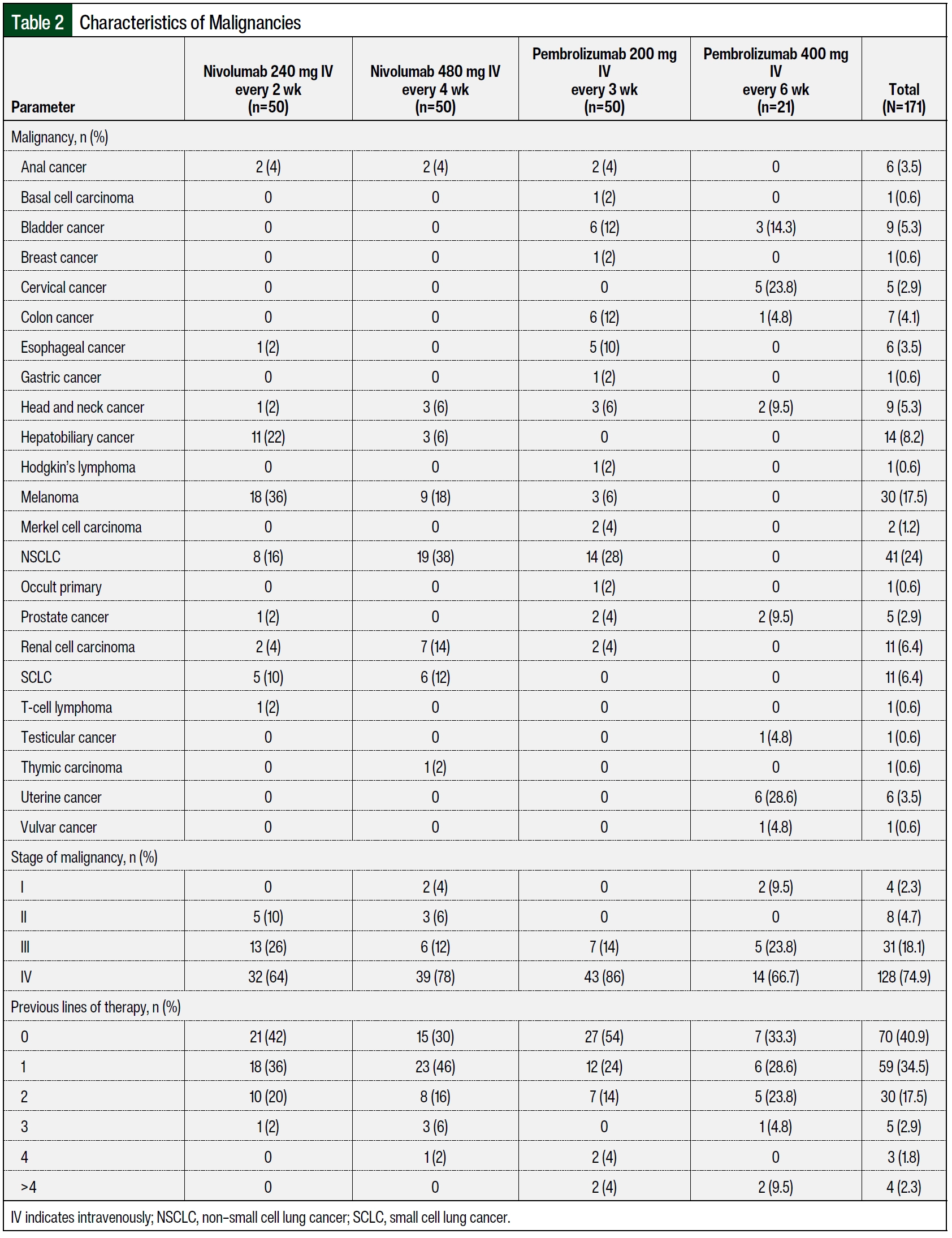
A total of 100 patients were included in the nivolumab arms, with 50 patients receiving nivolumab 240 mg IV every 2 weeks and 50 patients receiving nivolumab 480 mg IV every 4 weeks. The patients received ≥2 doses of nivolumab within 6 months of treatment initiation at DUHS during the study period and were included in the final analysis. The median ages in the groups that received nivolumab 240 mg IV every 2 weeks and nivolumab 480 mg IV every 4 weeks were 65 years and 68 years, respectively (Table 1). More patients in the group that received nivolumab 240 mg IV every 2 weeks were male (n=36 [72%] vs n=28 [56%], respectively) and were Black (n=10 [20%] vs n=5 [10%], respectively) than in the group that received nivolumab 480 mg IV every 4 weeks. The patients who received nivolumab 480 mg IV every 4 weeks had more comorbidities than those who received nivolumab 240 mg IV every 2 weeks (n=27 [54%] vs n=20 [40%], respectively). When comparing the nivolumab cohorts, more patients who received 480 mg IV every 4 weeks had chronic kidney disease (n=20 [40%] vs n=8 [16%]), and more patients who received 240 mg IV every 2 weeks had hepatitis (n=9 [18%] vs n=1 [2%]) and cirrhosis (n=6 [12%] vs n=3 [6%]). In the patients who received nivolumab 240 mg IV every 2 weeks, the most frequent malignancy was melanoma (n=18 [36%]), followed by hepatobiliary cancer (n=11 [22%]), non–small cell lung cancer (NSCLC; N=8 [16%]), and small cell lung cancer (SCLC; n=5 [10%]; Table 2). In the patients who received nivolumab 480 mg IV every 4 weeks, the most frequent malignancy was NSCLC (n=19 [38%]), followed by melanoma (n=9 [18%]), renal carcinoma (n=7 [14%]), and SCLC (n=6 [12%]). Most of the patients had stage III or stage IV malignancies. In comparing the nivolumab cohorts, more patients who received 480 mg IV every 4 weeks had stage IV cancer (n=39 [78%] vs n=32 [64%]), and more patients who received 240 mg IV every 2 weeks had stage III cancer (n=13 [26%] vs n=6 [12%]).
A total of 71 patients received pembrolizumab, with 50 patients receiving pembrolizumab 200 mg IV every 3 weeks and 21 patients receiving pembrolizumab 400 mg IV every 6 weeks group. The patients received at least 2 doses of pembrolizumab within 6 months of treatment initiation at DUHS during the study period and were included in the final analysis. The median age was 69 years for patients who had pembrolizumab 200 mg IV every 3 weeks and 62 years for those who received pembrolizumab 400 mg IV every 6 weeks (Table 1). A higher percentage of patients who received pembrolizumab 200 mg IV every 3 weeks were male (48% [n=24] vs 38.1% [n=8], respectively) and Black (24% [n=12] vs 9.5% [n=2], respectively) compared with those who received pembrolizumab 400 mg IV every 6 weeks. A higher percentage of patients in the arm that received pembrolizumab 400 mg IV every 6 weeks had comorbidities compared with the arm that received pembrolizumab 200 mg IV every 3 weeks (57.1% [n=12] vs 38% [n=19], respectively.
Specifically, when comparing the pembrolizumab cohorts, a higher percentage of patients who received 400 mg IV every 6 weeks had chronic kidney disease compared with those who received 200 mg IV every 3 weeks (52.4% [n=11] vs 28% [n=14], respectively; Table 1). In the patients who received pembrolizumab 200 mg IV every 3 weeks, the most frequent malignancy was NSCLC (n=14 [28%]), followed by colon cancer (n=6 [12%]), bladder cancer (N=6 [12%]), and esophageal cancer (n=5 [10%]; Table 2). In the patients who received pembrolizumab 400 mg IV every 6 weeks, the most frequent malignancy was uterine cancer (n=6 [28.6%]), followed by cervical cancer (n=5 [23.8%]) and bladder cancer (n=3 [14.3%]). The majority of the patients had stage III or IV malignancies. A higher percentage of patients who received pembrolizumab 200 mg IV every 3 weeks had stage IV cancer (86% [n=43] vs 66.7% [n=14], respectively), and a higher percentage of patients who received pembrolizumab 400 mg IV every 6 weeks had stage III cancer (23.8% [n=5] vs 14% [n=7], respectively).
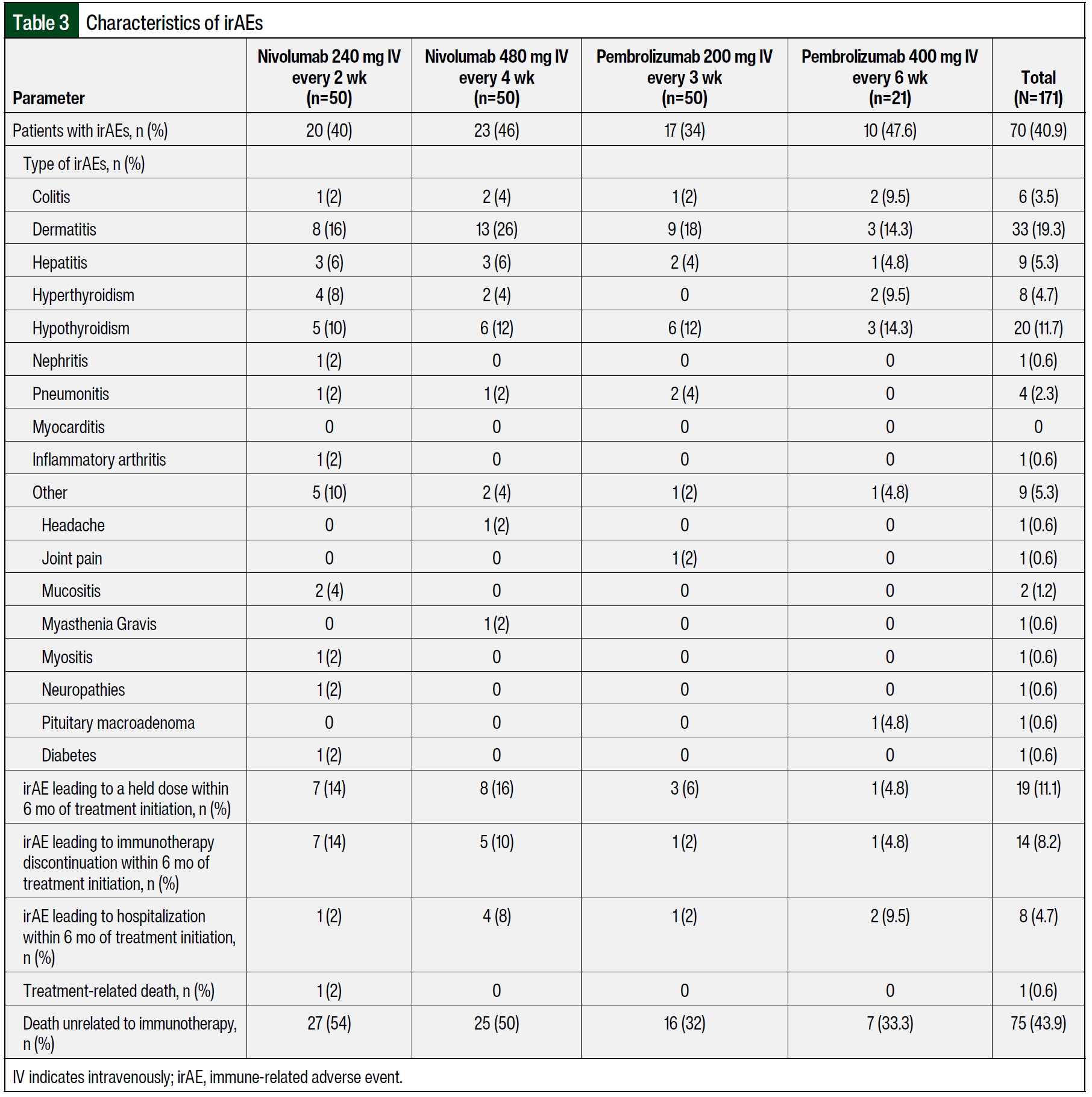
The incidences of irAEs were 40% (n=20) in the group that received nivolumab 240 mg IV every 2 weeks and 46% (n=23) in the group that received nivolumab 480 mg IV every 4 weeks (Table 3). The difference between the 2 groups was not significantly different (P=.54, chi-square test). The incidences of irAEs were 34% (n=17) in the group that received pembrolizumab 200 mg IV every 3 weeks group and 47.6% (n=10) in the group that received pembrolizumab 400 mg IV every 6 weeks. These differences in the incidences of irAEs between the dosing groups were not statistically significant (P=.28, chi-square test).
In the patients who received nivolumab 240 mg IV every 2 weeks, the most frequent irAEs were dermatitis (n=8 [16%]), followed by hypothyroidism (n=5 [10%]), hyperthyroidism (N=4 [8%]), and hepatitis (n=3 [6%]; Table 3). In the patients who received nivolumab 480 mg IV every 4 weeks, the most frequent irAEs were dermatitis (n=13 [26%]), followed by hypothyroidism (n=6 [12%]) and hepatitis (n=3 [6%]). The percentages of patients who required a dose to be held because of an irAE were comparable between the group that received nivolumab 240 mg IV every 2 weeks and the group that received nivolumab 480 mg IV every 4 weeks (35% [n=7] vs 34.8% [n=8], respectively). However, the cohort that received nivolumab 240 mg IV every 2 weeks had a higher percentage of patients with treatment discontinuation as a result of irAEs (35% [n=7] vs 21.7% [n=5], respectively), whereas the cohort that received nivolumab 480 mg IV every 4 weeks had a higher percentage of hospitalized patients as a result of irAEs (17.4% [n=4] vs 5% [n=1], respectively). One patient from the group that received nivolumab 240 mg IV every 2 weeks had treatment-related death, which was secondary to pneumonitis.
For patients who received pembrolizumab 200 mg IV every 3 weeks, the most frequent irAE was dermatitis (n=9 [18%]), followed by hypothyroidism (n=6 [12%]; Table 3). For the patients who received pembrolizumab 400 mg IV every 6 weeks, the most frequent irAE was dermatitis (n=3 [14.3%]), followed by hypothyroidism (n=3 [14.3%]) and colitis (n=2 [9.5%]). The percentage of patients who required a dose to be held because of an irAE was higher in the cohort that received pembrolizumab 200 mg IV every 3 weeks than in the cohort that received pembrolizumab 400 mg IV every 6 weeks (17.6% [n=3] vs 10% [n=1], respectively). Furthermore, the group that received pembrolizumab 400 mg IV every 6 weeks had a higher percentage of patients who discontinued treatment (10% [n=1] vs 5.9% [n=1], respectively) and who were hospitalized (20% [n=2] vs 5.9% [n=1], respectively). None of the patients in the pembrolizumab groups had treatment-related death.
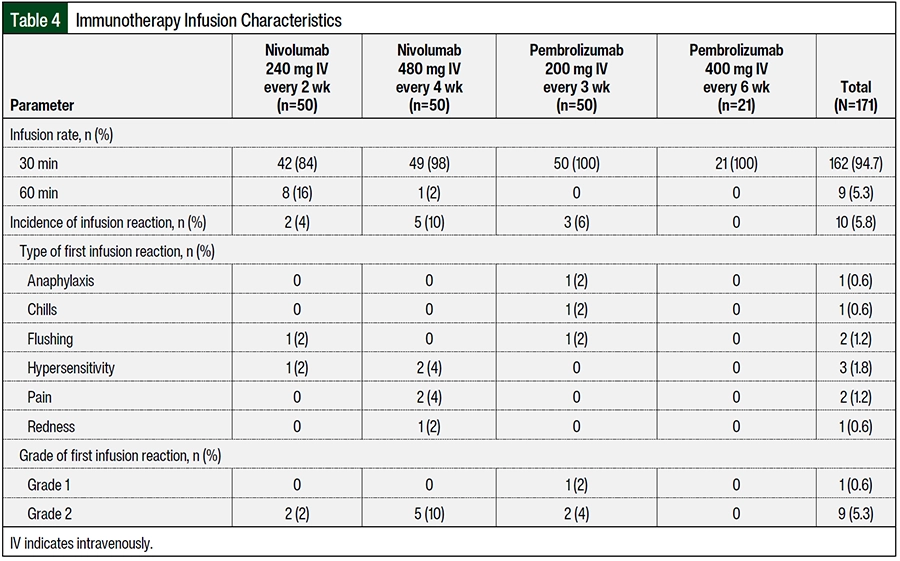
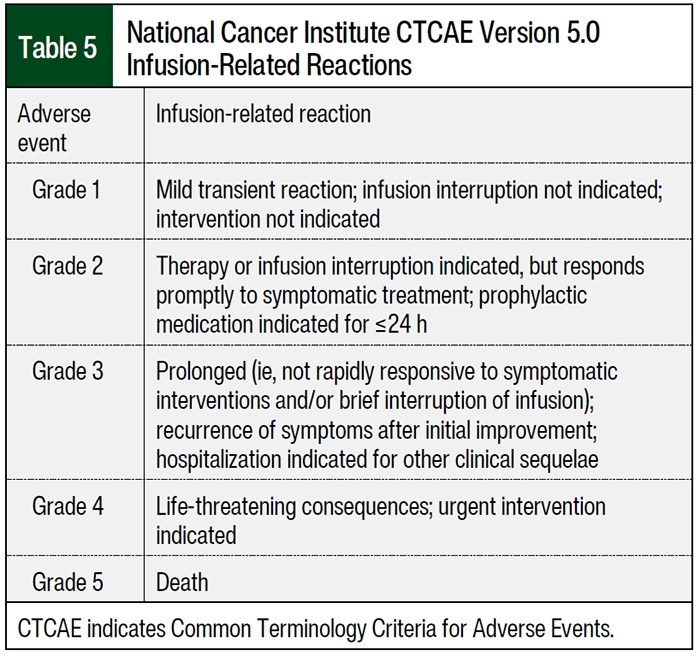
The patients who received nivolumab 480 mg IV every 4 weeks had a higher percentage of infusion reactions than those who received nivolumab 240 mg IV every 2 weeks (10% [n=5] vs 4% [n=2], respectively; Table 4). The patients who received nivolumab 240 mg IV every 2 weeks had infusion reactions of flushing and hypersensitivity and patients who received nivolumab 480 mg IV every 4 weeks had infusion reactions of hypersensitivity, pain, and redness. All patients had grade 2 infusion reaction according to the National Cancer Institute Common Terminology Criteria for Adverse Events (Table 5). The patients who received pembrolizumab 200 mg IV every 3 weeks had a higher percentage of infusion reactions than those who received pembrolizumab 400 mg IV every 6 weeks (6% [n=3] vs 0% [n=0], respectively), including anaphylaxis, chills, and flushing; of these 3 patients who had infusion reactions, 2 patients had a grade 2 reactions and 1 patient had a grade 1 reaction. A higher percentage of patients in the cohort that received nivolumab 480 mg IV every 4 weeks had an infusion time of 30 minutes (98% [n=49] vs 84% [n=42], respectively) than the patients in the cohort that received nivolumab 240 mg IV every 2 weeks. All of the patients who received pembrolizumab had an infusion rate of 30 minutes (Table 4).
Discussion
A new dosing regimen for nivolumab 480 mg IV every 4 weeks and pembrolizumab 600 mg IV every 6 weeks received accelerated FDA approval in 2018 and 2020, respectively.8,9,12,17 These approvals were based on pharmacokinetic modeling and exposure-response analyses that compared the predicted exposure of extended dosing schedules for nivolumab and pembrolizumab with those for previously approved dosing regimens.7,10-12 Population pharmacokinetic modeling and simulation studies have been reliably used to predict the exposure-response relationship of immune checkpoint inhibitors such as pembrolizumab12 and nivolumab.18 The flat dose exposure-response relationship has been well established for pembrolizumab19-22 and nivolumab23,24 across a wide range of malignancies. These model-based approaches were further used to evaluate the extended-interval frequencies of nivolumab and pembrolizumab.
The initial study with nivolumab compared standard schedules of 3 mg/kg every 2 weeks and nivolumab 240 mg IV every 2 weeks with nivolumab 480 mg IV every 4 weeks.6 The study used surrogate markers for efficacy (trough concentration [Cmin] and time-averaged concentration) and safety (peak concentration [Cmax]).6 Nivolumab pharmacokinetic exposure for the nivolumab regimen of 480 mg IV every 4 weeks was simulated for 3817 patients across various tumor types and was compared with that of 3 mg/kg every 2 weeks and nivolumab 240 mg IV every 2 weeks.6 Compared with 3 mg/kg every 2 weeks, nivolumab 480 mg IV every 4 weeks has a similar time-averaged concentration, which was approximately 16% lower Cmin, and 45% higher Cmax at steady state.6 These results suggest comparable exposure and safety profile of the different dosing schedules.
Similarly, another model-based study simulated pembrolizumab concentration-time profiles using population pharmacokinetic data from 2993 participants across multiple tumor types to compare the efficacy and safety of pembrolizumab 400 mg IV every 6 weeks with pembrolizumab 200 mg IV every 3 weeks and pembrolizumab 2 mg/kg every 3 weeks.10 The study showed that pembrolizumab 400 mg IV every 6 weeks had similar predicted exposure as the pembrolizumab 200 mg IV every 3 weeks and 2 mg/kg every 3 weeks.10
Based on primary studies with the initially approved dosing regimens, the prescribing information for nivolumab and pembrolizumab list the rate of infusion-related reactions as <1% and 0.2%, respectively.2,3 The safety profile of nivolumab 480 mg IV every 4 weeks has been assessed by pooling data from 61 patients who transitioned to this dose from a dose of 3 mg/kg IV every 2 weeks during 4 phase 3 clinical trials.6 The incidence of treatment-related adverse events in these patients after transitioning was comparable with that reported during the nivolumab weight-based treatment phase, and was consistent across all studies.6,12
Furthermore, in an ongoing randomized clinical trial (CheckMate 384), treatment with nivolumab 480 mg IV every 4 weeks versus with nivolumab 240 mg IV every 2 weeks over a 30-minute infusion were evaluated in patients with advanced NSCLC who previously received nivolumab 240 mg IV every 2 weeks.25 An interim analysis of 329 patients (166 patients received nivolumab 480 mg IV every 4 weeks and 163 patients received nivolumab 240 mg IV every 2 weeks) revealed comparable safety and efficacy at a median follow-up of 9.5 months (nivolumab every 4 weeks) and 10.2 months (nivolumab every 2 weeks).25 Any-grade treatment-related adverse events and those leading to treatment discontinuation were observed in 48% and 6%, respectively, of patients who received nivolumab 480 mg IV every 4 weeks versus 61% and 9%, respectively, in those who received nivolumab 240 mg IV every 2 weeks.12
To the best of our knowledge, our study is the first to compare the incidence of irAEs and infusion reactions in patients receiving nivolumab 240 mg IV every 2 weeks with those receiving nivolumab 480 mg IV every 4 weeks, and pembrolizumab 200 mg IV every 3 weeks versus pembrolizumab 400 mg IV every 6 weeks. Statistical analyses show that there were no significant differences in the incidence of any-grade irAEs in patients receiving nivolumab 240 mg IV every 2 weeks compared with patients receiving nivolumab 480 mg IV every 4 weeks (40% [n=20] and 46% [n=23], respectively; P=.54, chi-square test). Similarly, there were no significant differences in the incidence of any-grade irAEs among the pembrolizumab groups (pembrolizumab 200 mg IV every 3 weeks, 34% [n=17] vs pembrolizumab 400 mg IV every 6 weeks, 47.6% [n=10]; P=.28, chi-square test). In comparing the incidence of infusion reactions, patients who received nivolumab 480 mg IV every 4 weeks had a higher percentage of infusion reactions than those who received nivolumab 240 mg IV every 2 weeks (10% [n=5] vs 4% [n=2], respectively). Patients who received pembrolizumab 200 mg IV every 3 weeks had a higher percentage of infusion reactions than those who received pembrolizumab 400 mg IV every 6 weeks (6% [n=3] vs 0% [n=0], respectively). Overall, this study did not reveal any significant differences in irAEs and infusions reactions between the 2 dosing groups for nivolumab or pembrolizumab.
Limitations
One of the limitations of our study is the relatively small sample size, which limited our ability to have enough power to detect a statistical significance in the incidence of irAEs and infusion reactions between the different dosing schedules of the immunotherapies, if it exists. However, our findings still provide valuable information regarding the incidence of irAEs and infusion reactions that no previous study has evaluated. Despite the relatively low sample size in the cohort that received pembrolizumab 400 mg IV every 6 weeks, our findings show that the 2 dosing schedules might be comparable in terms of their safety profile.
Another limitation of our study is the duration of follow-up of 6 months. Although there were no significant differences in the rates of irAEs among patients who received nivolumab 240 mg IV every 2 weeks versus nivolumab 480 mg IV every 4 weeks and pembrolizumab 200 mg IV every 3 weeks versus pembrolizumab 400 mg IV every 6 weeks, we may be missing differences in the incidence and rates of late-onset irAEs that could occur outside our 6-month study window. However, we know that most incidences of irAEs occur within 14.8 weeks after the first dose of an immunotherapy agent. Our study was conducted as a retrospective chart review, which poses its own challenges because the incidence of irAEs might not be accurately reported in the chart.
Last, our study duration was expanded through June 30, 2021, to increase our sample size in the group that received pembrolizumab 400 mg IV every 6 weeks. At the time of the study expansion, the remaining 3 groups had reached the prespecified sample population of 50 patients and, as such, no further patients were included in those groups; this could have potentially introduced selection bias. The findings of this study add to the growing literature on the use of immunotherapy agents in cancer care. Future prospective, large-scale trials are warranted to validate the comparable safety of the different dosing schedules for pembrolizumab and nivolumab in patients with cancer.
Conclusion
We compared the incidence of irAEs and infusion reactions between the different FDA-approved dosing schedules for nivolumab 240 mg IV every 2 weeks versus nivolumab 480 mg IV every 4 weeks and pembrolizumab 200 mg IV every 3 weeks versus pembrolizumab 400 mg IV every 6 weeks. Our results show that the incidence of irAEs were not significantly different between the various dosing schedules in the nivolumab and pembrolizumab groups. The incidence of infusion reactions was higher in the patients who received nivolumab 480 mg IV every 4 weeks and in those who received pembrolizumab 200 mg IV every 3 weeks.
The results of this study offer real-world incidences of irAEs and infusion reactions among adults receiving nivolumab or pembrolizumab at DUHS between January 1, 2018, and June 30, 2021. Our results add to the scarce literature comparing the recently approved dosing schedules and the traditional dosing schedules of 2 of the most highly prescribed immunotherapy agents. These results can aid clinicians in deciding between the different dosing schedules, especially in the wake of a pandemic. Further studies with a large sample size are warranted to validate these results.
Source of Funding
The Duke BERD Methods Core’s support of this project was made possible in part by Grant Number UL1TR002553 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research (Dr Liu).
Author Disclosure Statement
Dr Brown is on the Speaker’s Bureau of Epizyme; Dr Salama has done research for Bristol Myers Squibb, Ideaya, Immunocore, Merck, Replimune, and Seagen, and is on the Scientific Advisory Board for Iovance, Novartis, Pfizer, and Regeneron; Dr Hanks has received honoraria from Merck and Novartis, has received research support from Exicure Therapeutics, Leap Therapeutics, Merck, Sanofi, and Tempest Therapeutics, and is a consultant to and is on the Scientific Advisory Board of G1 Therapeutics; Dr Karimi, Dr Liu, and Dr Erkanli have no conflicts of interest to report.
References
- Liu C, Seeram NP, Ma H. Small molecule inhibitors against PD-1/PD-L1 immune checkpoints and current methodologies for their development: a review. Cancer Cell Int. 2021;21:239.
- Opdivo (nivolumab) injection, for intravenous use [prescribing information]. Bristol Myers Squibb; October 2023. Accessed February 15, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/125554Orig1s121lbl.pdf
- Keytruda (pembrolizumab) injection, for intravenous use [prescribing information]. Merck; January 2024. Accessed February 15, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2024/125514s124lbl.pdf
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Long GV, Tykodi SS, Schneider JG, et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol. 2018;29:2208-2213.
- Zhao X, Shen J, Ivaturi V, et al. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann Oncol. 2020;31:302-309.
- Bristol Myers Squibb. Bristol-Myers Squibb’s Opdivo (nivolumab) Now the First and Only FDA-Approved PD-1 Inhibitor to Offer Every Four-Week Dosing. March 6, 2018. Accessed February 16, 2024. news.bms.com/news/corporate-financial/2018/Bristol-Myers-Squibbs-Opdivo-nivolumab-Now-the-First-and-Only-FDA-Approved-PD-1-Inhibitor-to-Offer-Every-Four-Week-Dosing/default.aspx
- US Food & Drug Administration. FDA approves new dosing regimen for pembrolizumab. April 29, 2020. Accessed February 16, 2024. www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-new-dosing-regimen-pembrolizumab
- Lala M, Li TR, de Alwis DP, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68-75. Erratum in: Eur J Cancer. 2021;144:400.
- Lala M, Akala O, Chartash E, et al. Pembrolizumab 400 mg Q6W dosing: first clinical outcomes data from KEYNOTE-555 cohort B in metastatic melanoma patients. Cancer Res. 2020;80(16 suppl):Abstract CT042.
- Turner DC, Kondic AG, Anderson KM, et al. Pembrolizumab exposure-response assessments challenged by Association of Cancer Cachexia and Catabolic Clearance. Clin Cancer Res. 2018;24:5841-5849.
- Tang SQ, Tang LL, Mao YP, et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021;53:339-354.
- Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:P1217-P1238.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Management of Immunotherapy-Related Toxicities. Version 1.2024. December 7, 2023. Accessed February 16, 2024. www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226.
- Bi Y, Liu J, Furmanski B, et al. Model-informed drug development approach supporting approval of the 4-week (Q4W) dosing schedule for nivolumab (Opdivo) across multiple indications: a regulatory perspective. Ann Oncol. 2019;30:644-651.
- Bellesoeur A, Ollier E, Allard M, et al. Is there an exposure-response relationship for nivolumab in real-world NSCLC patients? Cancers (Basel). 2019;11:1784.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550.
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286-4293.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- Chatterjee MS, Elassaiss-Schaap J, Lindauer A, et al. Population pharmacokinetic/pharmacodynamic modeling of tumor size dynamics in pembrolizumab-treated advanced melanoma. CPT Pharmacometrics Syst Pharmacol. 2017;6:29-39.
- Feng Y, Wang X, Bajaj G, et al. Nivolumab exposure-response analyses of efficacy and safety in previously treated squamous or nonsquamous non-small cell lung cancer. Clin Cancer Res. 2017;23:5394-5405.
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430-1437.
- Garon EB, Reinmuth N, Falchero L, et al. CheckMate 384: Phase IIIb/IV trial of nivolumab (nivo) 480 mg Q4W versus 240 mg Q2W after ≤ 12 months of nivo in previously treated advanced NSCLC. J Clin Oncol. 2019;37:100.