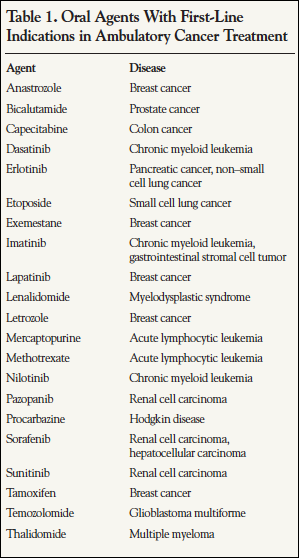
The predominant scenario for systemic treatment of cancer has traditionally involved administration of intravenous chemotherapy by highly trained personnel who closely monitored the patient. When these procedures took place in an oncologist’s office or in a hospital infusion center, extensive education of the patient and family was possible. More recently, however, an increasingly common situation involves the use of one or more oral medications and self-administered subcutaneous therapies in the home environment. The direct responsibility for drug acquisition and administration is shifting to the patients and their social support network, if available. At the present time, more than 20 oral medications are FDA approved for the first-line treatment of cancer (Table 1). In addition, a number of other oral agents are used for tumors that have relapsed or are refractory to initial treatment. According to the National Comprehensive Cancer Network, approximately 25% of all compounds in the oncology research and development pipeline are administered orally, so the trend is likely to continue.1
With this shift in responsibility comes the increased possibility that anticancer medications may not be administered correctly, especially for regimens that require repeated dosing. Overall estimates of adherence to long-term oral medication regimens range from 17% to 80%, with an average around 50%.2-4 A common assumption that adherence to oral anticancer agents would be higher, due to the severity of the disease, has been proven untrue. Studies indicate the adherence rates for cancer therapy are 15% to 97%.5 Nonadherence has been associated with worse outcomes in a number of disease states and with increased physician visits, higher hospitalization rates, longer hospital stays, disease worsening, and increased mortality.6 Approximately one-third to two-thirds of all medication-related hospitalizations are due to medication nonadherence—at a cost of $100 billion annually.7 The purpose of this article is to describe general concepts regarding patient adherence and the research related to adherence to cancer treatment. The incidence, risk factors, and consequences of this problem will be reviewed. The last article in this series will subsequently examine the best practices for maximizing adherence and clinical outcomes.
Definitions and Measures of Adherence
Adherence was defined by the World Health Organization in 2003 as the “extent to which a person’s behavior, taking medication, following a diet, and/or executing lifestyle changes corresponds with agreed recommendations from a healthcare provider.”4 Before that time, the term “compliance” had been more frequently used, but adherence is the term preferred by many authors, having a more positive connotation that places the patient in the role as an active participant. The International Society of Pharmacoeconomics and Outcomes Research convened a Medication Compliance and Persistence Work Group to develop standard definitions. Medication compliance was defined as “the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen.”8 Medication persistence was defined as “the duration of time from initiation to discontinuation of therapy.” No single term combines both of these characteristics. Some authors urge caution that applying any of these terms to an individual patient who does not take every dose correctly can stigmatize the patient and influence future relationships with healthcare providers.7
Techniques used to measure adherence have included indirect methods such as
- Self-report via interview, querying the patient about how easy it is for him or her to take prescribed medication, questionnaires, and diaries
- Clinical disease response
- Pill counts
- Automated pharmacy claims databases
- The Medication Event Monitoring System (MEMS, a microelectronic prescription vial cap that records openings)
Many consider MEMS to be the gold standard for adherence as it records the date and time of all vial openings.9 However, it is a costly method typically used only in a research setting, and it cannot detect whether one or more doses are removed from the vial or if a dose is actually ingested. Most studies have analyzed MEMS for one medication only, whereas most cancer patients receive multiple medications. Self-report methods have the advantage of being low cost, fast, and usually simple to complete. They can also be used for multiple concurrent medications. Oral interviews are reliant upon the availability of adequate time in the clinical setting and upon satisfactory communication between patient and clinician. Written instruments are limited by patient motivation and memory; in many cases, they are completed retrospectively, and studies have found that 30% of diary entries do not coincide with MEMS data. Clinical responses can be influenced by disease refractoriness as well as by medication adherence. Pill counts can be manipulated by the patient and do not reveal details of medication-taking behavior over time. Automated pharmacy claims databases can be used to identify medication use patterns, including persistence, switching, and discontinuation, in a large population of users in a timely manner.10 They are more likely than clinical trials to reflect real-world use patterns. Commonly used outputs from claims database analysis include the medication possession ratio (MPR), which compares the number of days medication was dispensed with the actual elapsed time, discontinuation/continuation, switching, medication gaps, and refill compliance or failure. Only direct pharmacokinetic and pharmacodynamic metrics can confirm ingestion of medication, but even these research methods are imprecise. In oncology trials, metabolite levels of mercaptopurine and corticosteroids have been assessed, as well as pharmacodynamics response using dehydroepiandrosterone (DHEA) sulfate suppression for prednisone adherence.7,9
Although there are many methods for estimating adherence, in both the clinical setting and for research, there is no consensus on a threshold level that is predictive of poorer outcomes. If therapeutic clinical trials do not assess adherence, it is difficult to predict whether nonadherence in the “real world” will result in a different outcome. Osterberg and colleagues have described the property of pharmacologic “forgiveness,” in which, based on interplay of pharmacokinetics and disease biology, certain deficits in adherence may or may not be clinically relevant.11 Many studies have arbitrarily set a utilization or MPR of 80% or higher as defining adherence. This provides a degree of consistency for evaluating the magnitude and frequency of the adherence problem but may not be appropriate for all diseases or all drug classes.
Adherence in Breast Cancer
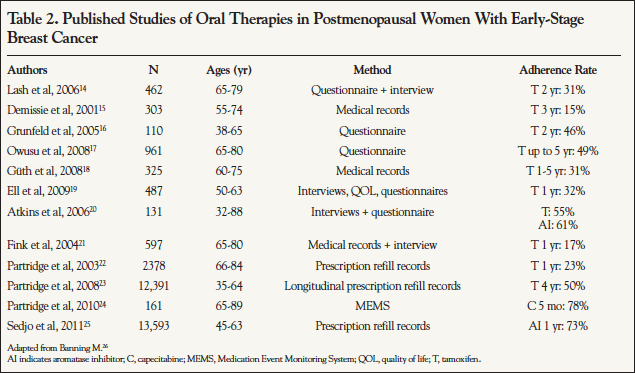
The most robust body of literature on adherence to cancer treatment involves adjuvant hormonal treatment (AHT) of early-stage, estrogen receptor–positive breast cancer. After surgical resection, with or without adjuvant chemotherapy, hormonally targeted therapy for 5 years decreases the risk of death by about 30%.12 The antiestrogen tamoxifen was the first agent used for this purpose, and the aromatase inhibitors anastrozole, letrozole, and exemestane are more recent options that are slightly more effective in postmenopausal women. Table 2 summarizes the largest evaluations of adherence to therapy for early-stage breast cancer through 2011. Adherence rates are alarmingly low, ranging from 15% to 73%, depending upon the class of drug and time point evaluated. A number of these studies based on self- report may even overestimate adherence based on a prospective comparison, which found self-report and pill counts statistically significantly overestimate the degree to which patients adhered to their tamoxifen regimen, compared with data recorded by a MEMS vial.13
Additional in-depth information about AHT adherence patterns was published by Nekhlyudov and colleagues, who identified 2207 women from a single health plan who were diagnosed with early-stage breast cancer and who initiated therapy between 2000 and 2005.27 At the end of the first year of treatment, 79% remained on therapy without a gap exceeding 60 days and 85% without a gap exceeding 180 days. However, by year 5, only 27% and 29% remained without 60- and 180-day gaps, respectively. Age ≥70 years (vs <50 years) was consistently associated with an increased likelihood of treatment gaps. Longer gaps were associated with a lower likelihood of resuming therapy. Another study found that age ≥65 years, having a nononcologist write the prescription, and having a larger number of other prescriptions and a higher copayment were associated with nonpersistence.28 Compared with a copayment of <$30, a copayment of $30.00 to $89.99 was associated with nonpersistence in older women (odds ratio, 0.69) but not in younger women, whereas a copayment of $90 or more affected both groups (odds ratio, 0.72 and 0.82, respectively).
Nonadherence to AHT has documented associations with breast cancer outcomes. A large HMO database was used to identify 8769 patients with a prescription for AHT.29 Therapy was discontinued early (defined as >180 days elapsed from prior prescription) in 31%, and of those who continued therapy, 28% were nonadherent (MPR <80%). Estimated survival at 10 years was 80.7% for those who continued therapy versus 73.6% for those who had discontinued it (P<.001). Those who were adherent also had a higher 10-year survival rate than those who were not (81.7% vs 77.9%, respectively, P<.001). If a 90% cut-off for MPR was used, the survival impact was not observed. This result was similar to a trial that analyzed all women prescribed tamoxifen in a defined population in Scotland (N=1633). Mortality was improved when the duration of adherence was >2.4 years (hazard ratio, 0.85; 95% CI, 0.83-0.87) and worse when adherence was <80% (hazard ratio, 1.1; 95% CI, 1.001-1.21).30
Although lack of adherence with AHT in these trials was associated with poor survival, there may be other factors at play for both poor adherence and survival, such as physical condition, psychological outlook, and health behaviors, that also need attention by the healthcare community.
Adherence in Childhood Leukemia
The earliest research regarding adherence to antineoplastic agents was conducted in the pediatric population because oral agents such as corticosteroids, mercaptopurine, thioguanine, and methotrexate have been part of standard long-term treatment of acute lymphoblastic leukemia (ALL), a curable cancer, for over 30 years. The nonadherence rates have been observed in small studies to range from 10% to over 50%.31 A MEMS-based study found that 17% of patients used <80% of prescribed doses.32 Using metabolite levels of mercaptopurine, one study identified 2% of patients with ALL as completely nonadherent.33 Another study found that 52% of patients had no DHEA suppression, indicative of prednisone nonadherence.34 The same investigators noted that nonadherence with cotrimoxazole for infection prophylaxis (as documented by serum levels) seemed to be a proxy for nonadherence with anticancer medications.
The evidence for a detrimental impact of nonadherence on outcomes of ALL treatment is primarily indirect. Poorer outcomes have been associated with lower mercaptopurine metabolite levels. The relapse-free survival at 5 years was 63% in the group below the median and 84% in the group above the median (P=.0018).35,36
Children older than 11 years have been noted to be statistically significantly less adherent than younger children. Other sociodemographic variables do not seem to be predictive. Poorer adherence has been found in parent-adolescent pairs who lack concordance regarding medication instructions, effectiveness, and responsibilities for administration. Thus far, no studies have reported on adherence to other oral antineoplastic agents in children with cancer.
Adherence in Testicular Cancer
Another malignancy that occurs primarily in younger individuals and is curable with chemotherapy is testicular cancer. A study of 209 consecutive patients diagnosed with testicular cancer analyzed nonadherence to follow-up appointments (defined as 2 failures to attend at least 1 month apart, despite written reminders).37 Prior to initial treatment, the patients completed questionnaires regarding psychosocial factors and clinician interactions without explicitly being told of the planned analysis of follow-up behavior. There was no relationship of age, socioeconomic status, education, or cancer management method to the likelihood of nonadherence. However, there was a highly significant association between nonattendance and men’s perception of an unsatisfactory affective relationship with their clinician as measured on the Medical Interview Satisfaction Scale (MISS). Men with lower scores than the median were 3.1 times more at risk of nonattendance than those with higher scores (P=.005). Individual items in the Affective Scale of the MISS that were found to be significant were “I feel the doctors accept me as a person” and “The doctors are people I would trust with my life.” Analyses of therapy received and cancer-related outcomes were not reported in this study.
Adherence in Chronic Myeloid Leukemia
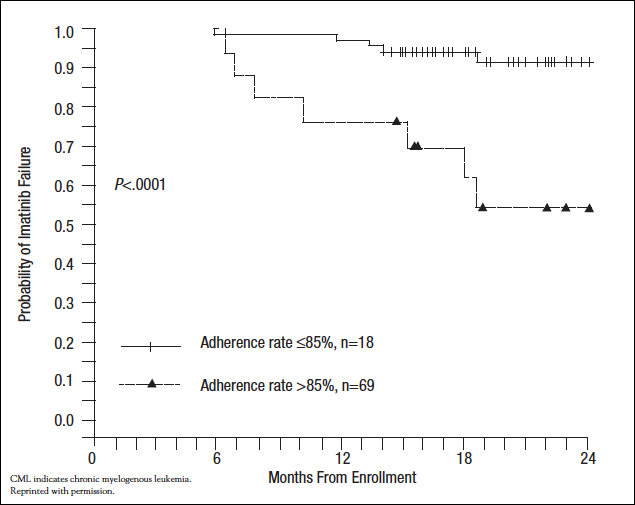
The vision of a treatment approach that specifically targets malignant cells was first realized with the development of imatinib, an inhibitor of the BCR-ABL tyrosine kinase, for chronic myelogenous leukemia (CML). Imatinib dramatically improved rates of major cytogenetic response compared with earlier therapies (87.1% vs 34.7%, respectively). Eight-year event-free survival is 81% and overall survival is 85%.38 Retrospective analyses have found adherence rates with imatinib for CML or gastrointestinal stromal cell tumors to be about 80%. A prospective trial in Belgium evaluated 169 patients with CML contributed by 51 oncologists over a 90-day period.39 The median time from diagnosis was 42 months. Adherence was determined based on pill counts. On average, patients with suboptimal response had statistically significantly higher mean percentages of imatinib not taken (23.2% ± 23.8%) than those with optimal response (7.3% ± 19.3%, P=.005). Complete cytogenetic response was also correlated with fewer treatment gaps during a 90-day period. British and French investigators enrolled 87 consecutive chronic-phase CML patients to measure adherence during a 3-month period using a MEMS device.40 After the monitoring period, the patients were followed for a median of 19 months during which BCR-ABL1 transcripts were measured at 6- to 12-week intervals and a rising level triggered bone marrow examination for cytogenetic relapse. The median adherence rate was 97.6% (range, 24%-103.8%). However, in the 18 patients (20.7%) with adherence ≤85%, the probability of losing complete cytogenetic response was 36.3%, compared with 1.4% in patients with higher adherence (P<.0001). Figure 1 shows the Kaplan-Meier analysis of this effect over time.
Adherence in Cancer Pain Syndromes
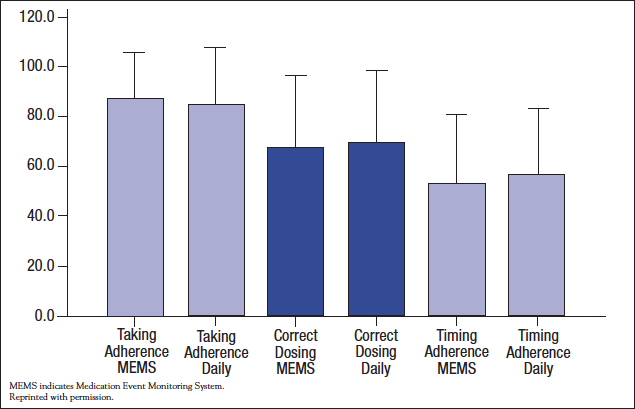
Many examples of adherence problems involve conditions that are relatively asymptomatic, but this is not always the case. Analgesics for cancer pain are taken in accordance with recommendations by only 20% to 90% of patients. One prospective study that compared MEMS with a diary found no difference between the assessment methods, but adherence rates for around-the-clock analgesics, even in this selected population, were disappointing (Figure 2).9 The higher rate for “correct dosing” than for “timing” indicates that some patients take the recommended amount per day, but at the wrong intervals.
Risk Factors for Nonadherence
A number of barriers and risk factors have been associated with poor adherence. Adverse events are the most obvious cause, and studies have shown that detection methods used in clinical trials and in routine practice underestimate their impact.41 Other barriers include forgetfulness, competing priorities, decisions to omit doses, lack of information, higher out-of-pocket costs, duration of treatment, a poor relationship with the healthcare provider, and emotional factors.7,25,31,42 Patients with a missed appointment or a higher level of comorbidity, those taking regimens with high dosing frequency, and those with lower out-of-pocket pharmacy costs in the year prior to a cancer diagnosis have been more likely to have nonadherent behavior.25,43,44 Interestingly, a meta-analysis found that patients in poor health secondary to a nonserious condition tend to have good adherence whereas those in poor health due to serious conditions have worse adherence.44 Patients who report themselves as having less influence over their own health more frequently forget their medication.20 Adherence is 1.5 times lower in patients from families in conflict.45 Some studies have had differing conclusions regarding age. For example, adolescents and young adults tend to be less adherent than younger children.31 The elderly have exhibited lower adherence rates in some studies, often attributed to cognitive deficits and complicated regimens, but in other situations, younger patients are more at risk.
Lack of direct communication about the importance of long-term orally administered cancer therapy and about barriers patients might be experiencing is likely an important component of nonadherence. For example, 14 oncologists were recruited to participate in a study of communication about breast cancer therapy but were not told that the specific focus was on adherence and persistence with AHT.46 Interviews with a total of 28 patients were video- and audio-recorded and the transcripts analyzed in a systematic manner.
Patients’ duration of time on AHT ranged from planned imminent initiation to 59 months. The study found that, overall, oncologists and patients had a good rapport but that the conversations did not significantly address adherence to therapy. Oncologists were not observed engaging the patient in a discussion of emotional or other barriers to continuing with therapy or taking therapy daily. No discussion took place explicitly linking adherence to “best outcomes” of therapy, and no oncologist discussed what to do if a dose was missed or asked patients if they foresaw any problems with adhering to therapy. The most common discussion relating to adherence to therapy was a “perfunctory review” of all medications taken, referred to in the analysis as a medication inventory. The likely time on therapy (persistence) was mentioned in 43% of visits but was usually done while describing study design and results and did not involve patients’ views on the challenges of such therapy. These findings show that little specific information was sought on medication adherence and indicate the lack of a basis for the oncologists’ stated beliefs that adherence was not an issue for their patients. The authors also noted that the conversations about AHT did not resemble those about chemotherapy, during which the cancer is still being “fought.” An apparent conundrum exists during the phase of AHT between the desire to be hopeful and supportive of life activities that approach normalcy and the need to encourage continued anticancer treatment.
Summary
Oral medications and self-administered subcutaneous therapies are increasingly important facets of cancer treatment. Even though they offer the patient convenience over intravenous infusions, oral medications and self-administered subcutaneous therapies are not necessarily less toxic. The amount of direct contact time the patient has with a healthcare professional is usually decreased, and the responsibility of daily decisions about administration of these critical medications has been transferred to the patient. Nonadherence is associated with worse outcomes in some cancers, but more research is clearly needed to assist clinicians in prioritizing areas for improvement.
References
- Schwartz RN, Eng KJ, Frieze DA, et al. NCCN Task Force Report: specialty pharmacy. J Natl Compr Canc Netw. 2010;8(suppl 4):S1-S12.
- Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22:313-356.
- Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652-661.
- Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35:207.
- Hohneker J, Shah-Mehta S, Brandt PS. Perspectives on adherence and persistence with oral medications for cancer treatment. J Oncol Pract. 2011;7:65-67.
- DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794-811.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;60:487-497.
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44-47.
- Oldenmenger WH, Echteld MA, de Wit R, et al. Analgesic adherence measurement in cancer patients: comparison between electronic monitoring and diary. J Pain Symptom Manage. 2007;34:639-647.
- Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565-574.
- Osterberg LG, Urquhart J, Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88:457-459.
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Version 2.2011. National Comprehensive Cancer Network Web site. www.consensocancermamario.com/lib/files/documents/uploaded/NCCN2011_BC.pdf. Accessed January 28, 2012.
- Waterhouse DM, Calzone KA, Mele C, et al. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189-1197.
- Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215-220.
- Demissie S, Silliman R, Lash TL. Adjuvant tamoxifen: predictors of use effects, and discontinuation in older women. J Clin Oncol. 2001;19:322-328.
- Grunfeld EA, Hunter MS, Sikka P, et al. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59:97-102.
- Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549-555.
- Güth U, Huang DJ, Schötzau A, et al. Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. Br J Cancer. 2008;99:428-433.
- Ell K, Vourlekis B, Xie B, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115:4606-4615.
- Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer. 2006;42:2271-2276.
- Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuation and non-adherence in older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2004;22:3309-3315.
- Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602-606.
- Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556-562.
- Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol. 2010;28:2418-2422.
- Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191-200.
- Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care. 2012;21:10-19.
- Nekhlyudov L, Li L, Ross-Degnan D, et al. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat. 2011;130:681-689.
- Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534-2542.
- Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529-537.
- McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763-1768.
- Landier W. Age span challenges: adherence in pediatric oncology. Sem Oncol Nurs. 2011;27:142-153.
- Lau RC, Matsui D, Greenberg M, et al. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;30:85-90.
- Lennard L, Welch J, Lilleyman JS. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: a possible indicator of non-compliance? Br J Cancer. 1995;72:1004-1006.
- Festa RS, Tamaroff MH, Chasalow F, et al. Therapeutic adherence to oral medication regimens by adolescents with cancer. I. Laboratory assessment. J Pediatr. 1992;120:807-811.
- Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989;7:1816-1823.
- Lilleyman JS, Lennard L. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet. 1994;343:1188-1190.
- Moynihan C, Norman AT, Barbachano Y, et al. Prospective study of factors predicting adherence to medical advice in men with testicular cancer. J Clin Oncol. 2009;27:2144-2150.
- NCCN Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia Version 2.2012. National Comprehensive Cancer Network Web site. http://guidelines.nccn.org/epc-guideline/guideline/id/EBA4F5EF-5A9B-E0B4-C6A4-BFDD8FA8BF78?jumpTo=false#. Accessed January 28, 2012.
- Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401-5411.
- Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733-3736.
- Oberguggenberger A, Hubalek M, Sztankay M, et al. Is the toxicity of adjuvant aromatase inhibitors underestimated? Complementary information from patient-reported outcomes (PROs). Breast Cancer Res Treat. 2011;128:553-561.
- Kirk MC, Parker B. Enhancing communication between oncologists and breast cancer patients. Breast Cancer Res Treat. 2007;106(suppl 1):S261-S262. Abstract 6043.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296-1310.
- DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care. 2007;45:521-528.
- DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207-218.
- Davidson B, Vogel V, Wickerham L. Oncologist-patient discussion of adjuvant hormonal therapy in breast cancer. Results of a linguistic study focusing on adherence and persistence to therapy. J Support Oncol. 2007;5:139-143.