Bevacizumab is a recombinant humanized monoclonal antibody that binds to vascular endothelial growth factor (VEGF)-A, inhibiting the activation of its receptors (FLT1 and KDR) on the surface of endothelial cells.1 This inhibition prevents the initiation of angiogenesis, which contributes to tumor growth and proliferation by providing a blood supply for malignant cells.2 Bevacizumab is US Food and Drug Administration approved for colorectal cancer, non–small-cell lung cancer (NSCLC), HER-2–negative breast cancer, glioblastoma, and renal-cell cancer. Some of the common adverse reactions of bevacizumab include hypertension, epistaxis, headache, rhinitis, stomatitis, asthenia, proteinuria, taste alteration, dry skin, rectal hemorrhage, back pain, and exfoliative dermatitis.1
Reversible posterior leukoencephalopathy syndrome (RPLS) is a rare adverse event reported with the use of bevacizumab. In clinical studies, the incidence has been less than 0.1% and the onset of symptoms has occurred from 16 hours to 1 year after initiation.1 This clinicalradiologic syndrome is characterized by headache, seizures, altered consciousness, visual disturbances including cortical blindness, and radiographic abnormalities suggesting subcortical white matter edema of the posterior hemispheres.3-6
Some of the risk factors associated with the incidence of RPLS include acute elevations of blood pressure (such as eclampsia), fluid overload, renal failure, and exposure to immunosuppressants, colony-stimulating factors, or cytotoxic drug therapy.2-5 Because there was some disagreement on whether the syndrome always represented a leukoencephalopathy, Casey and colleagues proposed the term “posterior reversible encephalopathy syndrome” in 2000.7 The term “hyperperfusion encephalopathy” has also been suggested, because the condition is not always reversible.8
This present case describes the presence of RPLS in a patient with NSCLC who developed the symptoms hours after undergoing monotherapy with bevacizumab.
Case Report
A 77-year-old man presented to the outpatient oncology clinic to receive maintenance chemotherapy with bevacizumab for the treatment of stage IV NSCLC, which had been diagnosed 4 years prior. He had been initially treated with left upper lobe lobectomy, but because of recurrent metastatic disease found 2 years postsurgery, he received chemotherapy with bevacizumab, carboplatin, and paclitaxel for a period of 6 months. Because there was residual lung disease after chemotherapy, he was included in a phase 2 clinical trial using concurrent sunitinib and electron beam radiation therapy to the chest (5000 cGy). After completion of this protocol, he initiated bevacizumab monotherapy every 3 weeks for approximately 17 months. His medical history was also significant for hypothyroidism, dyslipidemia, hypertension, atrial fibrillation, and proteinuria secondary to the chronic use of bevacizumab. His home medications included nifedipine 25 mg daily, levothyroxine 125 mcg daily, niacin 500 mg daily, and warfarin with a total weekly dose of 25 mg.
On examination at the clinic visit, his vital signs were normal (blood pressure, 122/60 mm Hg; pulse, 50 bpm; temperature, 97.3°F [36.3°C]) and his only complaint was fatigue. The laboratory examinations were normal, except for an increase in his liver enzymes compared with our laboratory tests taken 7 days earlier (alkaline phosphatase increased from 88 U/L to 325 U/L, alanine aminotransferase [ALT] increased from 13 U/L to 93 U/L, and aspartate aminotransferase [AST] increased from 21 U/L to 45 U/L). The patient received 1165 mg (15 mg/kg, patient’s weight, 77.7 kg) of bevacizumab in 100 mL of normal saline which was infused over a period of 30 minutes. Before the infusion, he received 25 mg of diphenhydramine intravenously as premedication and 1000 mL of normal saline for hydration.
Twenty-four hours after the infusion of bevacizumab, the patient presented with disorientation along with difficulty of ambulation. The movement of all 4 extremities was not affected and no acute changes in vision, speech, facial expressions, or facial symmetry were noted. He was brought to the emergency department, where he was found to be hypertensive (221/73 mm Hg), his mental status was altered, and he was experiencing lightheadedness and decreased responsiveness. His first electrocardiogram showed marked sinus bradycardia, with a ventricular rate of 44 bpm. The results of the laboratory tests conducted on admission were within normal limits, except for the consistent elevation of alkaline phosphatase and ALT (290 U/L and 69 U/L, respectively). His blood pressure was initially managed with hydralazine 10 mg intravenous (IV) and subsequently with a nitroglycerin drip.
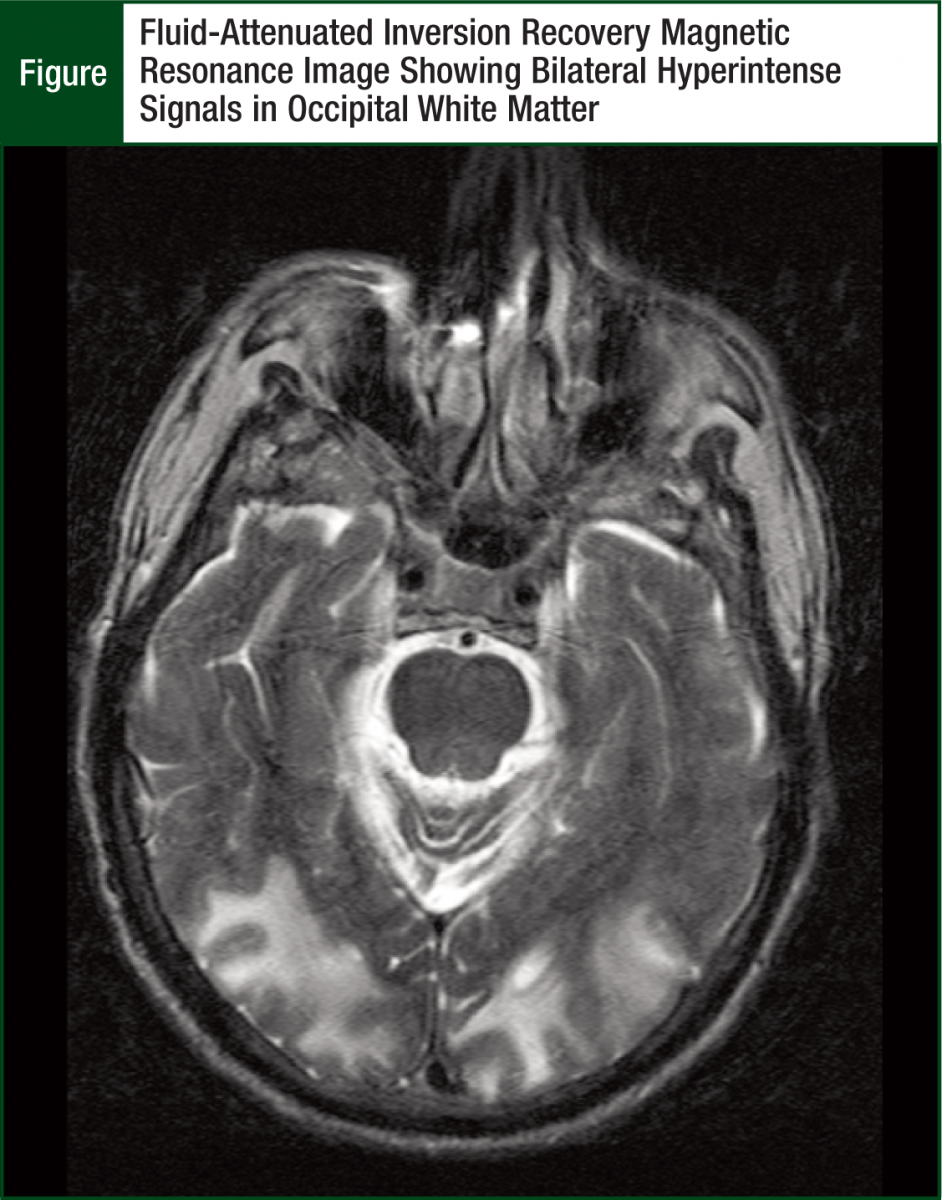
A computed tomography scan of the brain was performed, revealing cerebral atrophy and attenuation zones in the parietal lobes, temporal occipital lobes, and the right cerebellum, without significant masses. The differential diagnosis included underlying edema from metastatic disease (although the absence of a mass made this possibility unlikely), periventricular leukomalacia from underlying small vessel disease, or hypertensive encephalopathy. However, the brain magnetic resonance imaging (MRI) obtained after admission demonstrated radiologic findings suggestive of RPLS.
There were large areas of abnormal fluid-attenuated inversion recovery (FLAIR) signals involving the bilateral posterior parietal, occipital, and cerebellar hemispheres primarily involving the white matter. Some gray matter involvement was also identified. Cerebellar hemisphere lesions and areas of cortical enhancement seen within the left posterior parietal lobe and right occipital lobe were found suspicious for acute infarct, and no evidence of metastatic disease was observed (Figure).
A neurologic examination during the patient’s second day of admission revealed diplopia, presence of bilateral Babinski’s reflex, and possible right pronator drift. A urinalysis revealed worsening of proteinuria (500 mg/dL from a baseline of 182 mg/dL 1 month prior). His blood pressure remained controlled with amlodipine 10 mg daily, oral hydralazine 10 mg twice daily, and metoprolol 5 mg IV as needed. His mental status improved slightly, but he still appeared confused.
Examination on day 5 of hospitalization showed that his mental status had significantly improved, and he was awake and interactive. However, his vision was diffusely blurred on both eyes, and his reflexes were diminished bilaterally. His laboratory values remained stable during his hospital stay. The changes in his liver function tests improved. An abdominal ultrasound revealed that these changes were secondary to cholelithiasis. After discharge, the patient had gradual improvement of diplopia but continued to have difficulty reading words. Bevacizumab’s treatment was discontinued, and maintenance therapy was initiated with pemetrexed every 21 days. Fourteen months after the initial episode, the patient presented with similar symptoms, including altered mental status, left-sided flank pain, lethargy, visual hallucinations, bilateral blindness, and hypertension (218/95 mm Hg).
His only new medication besides pemetrexed was metoprolol 12.5 mg daily in place of nifedipine, and according to his family, his systolic home blood pressure readings were usually between 100 mm Hg and 110 mm Hg. Neurologic examination on admission was remarkable for altered mental status consistent with encephalopathy and bilateral cortical blindness. A repeat MRI was performed showing areas of abnormal white matter FLAIR signals in the bilateral posterior parietal, occipital, and cerebellar hemispheres that were slightly improved when compared with the MRI performed during the first episode. Encephalomalacia involving the bilateral occipital lobes that were thought to represent laminar necrosis from remote infarcts were observed. No evidence of acute infarct, mass, or hemorrhage was identified.
The patient’s laboratory tests were significant for elevated liver and pancreatic enzymes (alkaline phosphatase, 262 U/L; total bilirubin, 10.7 mg/dL; AST, 176 U/L; ALT, 222 U/L; amylase, 242; lipase, 205), and his urinalysis was negative for proteinuria. The results of an endoscopic retrograde cholangiopancreatography were normal. The management consisted of IV and oral antihypertensive medications, as well as broad-spectrum antibiotics because of the presence of bacteremia.
Discussion
This report describes a case of possible RPLS in a patient receiving maintenance therapy with bevacizumab. He developed some of the typical manifestations, including decreased levels of consciousness, confusion, and visual disturbances.4 The findings in his MRI were consistent with the characteristic radiographic changes of RPLS described in previous reports, indicating white matter involvement and bilateral abnormal FLAIR signals in the posterior circulation areas, especially the parietal and occipital lobes.4,7 The observed gray matter involvement and cerebellar neuroimaging abnormalities have also been reported but are considered atypical features of RPLS.3,4,9
Resolution of the clinical symptoms was observed within the 6 days of hospitalization, except for the visual disturbances, which required longer rehabilitation. This is consistent with the time to recovery previously published, which suggests a range of several days to weeks for the resolution of both clinical and MRI manifestations.3-5,9 The intracranial findings in the repeated neuroimaging study revealed that the radiologic lesions were not fully reversible.
Other RPLS cases have reported the presence of irreversible damage and the need of extended periods of time (up to 17 months) for a complete resolution of the initial findings.3,4,7-9 Residual infarcts have also been previously observed, but the improvement or resolution during follow-up studies suggested that the abnormalities were secondary to edema, and for other cases it was unknown whether the findings represented preexisting ischemia.4,9
Different explanations have been proposed to understand the pathophysiology of RPLS. One theory associates RPLS with a failure in the cerebral autoregulation to maintain a constant cerebral blood flow, regardless of changes in systemic blood pressure through the constriction and dilation of the arterioles. According to this hypothesis, spontaneous and severe elevations in blood pressure are not compensated, and the constricted arterioles dilate leading to brain hyperperfusion. The increased perfusion can result in the breakdown of the blood-brain barrier, which leads to extravasation of fluid, macromolecules, and red blood cells into the brain parenchyma.4,5,7
A second theory involving endothelial damage is often associated with drug-induced RPLS. This mechanism, although not clearly understood, can alter the vascular permeability of the blood-brain barrier and the systemic blood pressure, decreasing the threshold for the development of RPLS.3,5 The pathogenesis of RPLS secondary to bevacizumab appears to be related to those 2 theories, because bevacizumab not only has the ability to cause rapid elevations of blood pressure, but also causes VEGF alterations that may increase cerebral vascular permeability.2,3,5
Vascular endothelial dysfunction also appears to be the mechanism by which bevacizumab causes proteinuria and hypertension. A study comparing the amniotic fluid of healthy, diabetic, and preeclamptic women concluded that preeclampsia, a condition characterized by hypertension and proteinuria, was associated with increased levels of soluble FLT1 suggesting endothelial cell alterations.10 A different study conducted in mice indicated that IV infusion of anti-VEGF–neutralizing antibodies caused rapid glomerular endothelial cell detachment and hypertrophy, inducing the development of proteinuria.11 Similarly, 2 articles describing the management of bevacizumab’s toxicities affirmed that the binding inhibition of VEGF-A to VEGF-R impairs protein homeostasis by altering the permeability, development, and function of the glomerular vascular endothelium and the renal filtration system.2,6
After a review of current evidence, the Cardiovascular Toxicities Panel from the National Cancer Institute (NCI) concluded that the elevations in blood pressure caused by VEGF signaling pathway inhibitors were a result of increased peripheral resistance secondary to endothelial damage and decreased nitric oxide production.12
Using the Naranjo algorithm, the likelihood that the RPLS in the patient described in this report was caused by bevacizumab was estimated as probable, because the calculated score was 6. The acute elevation of blood pressure (221/73 mm Hg in the emergency department from 122/60 mm Hg before bevacizumab’s infusion) and the worsening of proteinuria (500 mg/dL from a baseline of 182 mg/dL 1 month earlier) suggest the incidence of endothelial cell dysfunction secondary to bevacizumab’s anti-VEGF activity, which, as described, may explain the development of RPLS.
Hypertension and proteinuria were present in another case of RPLS in a patient receiving chemotherapy with FOLFIRI (leucovorin, fluorouracil, irinotecan) plus bevacizumab, in which systemic endothelial cell dysfunction was also suspected.13 This case was included in a MEDLINE search that identified 24 cases of RPLS in patients treated with anticancer drugs. Five of these cases (21%) were associated with the use of bevacizumab and similar to our patient; all these patients receiving bevacizumab presented with elevations of blood pressure (systolic blood pressure range, 140-190 mm Hg), and the majority of them were managed with either oral or IV antihypertensive medications.3,13-17
Because systemic hypertension predisposes to the development of RPLS, Tam and colleagues have concluded that patients with cancer who have significant fluid overload, mean blood pressure more than 25% of baseline, and/or creatinine greater than 0.16 mmol/L are at high risk for the development of this syndrome.18 Similarly, Koopman and colleagues have recommended strict blood pressure control to prevent RPLS in patients undergoing therapy with bevacizumab and have suggested to withhold treatment when blood pressure reaches grade 2 severity or more based on the NCI Common Toxicity Criteria.19
In fact, the association between unmanaged hypertension and serious adverse events from endothelial growth factor signaling pathway inhibitors was one of the reasons the Investigational Drug Steering Committee of the NCI recently published the following recommendations for the management of blood pressure in patients receiving this type of chemotherapy agent12:
- Assess for potential cardiovascular complications before initiation of treatment, identify and address preexisting hypertension before initiation of therapy
- Monitor blood pressure weekly during the first cycle and at least every 2 to 3 weeks throughout treatment, set a blood pressure goal of <140/90 mm Hg or lower based on patients’ comorbidities
- Aggressively manage emergent hypertension to prevent the development of complications.
In addition to hypertension, different reviews of cases have identified other common features that may explain the increased incidence of RPLS. It appears to develop more often in females, and it has been found in patients as young as 12 years of age.3,9,20 The most common associated malignancies identified by Marinella and Market include lymphoma, colorectal cancer, and lung cancer.3 However, the authors conclude that because of the diversity of underlying malignancies, it is uncertain whether the biology of the tumor has a causal relationship; they have found a better correlation with the use of polychemotherapy and the emergence of new cytotoxic agents.3
Similarly, Vaughn and colleagues have suggested that the increased incidence of RPLS cases in patients with cancer was probably secondary to the toxic effects of most major classes of chemotherapy in the vascular endothelium.5 It is undetermined whether the use of the other chemotherapy agents might have put our patient at an increased risk for the development of this adverse event. Sunitinib has demonstrated anti-VEGF activity, it can cause hypertension similar to bevacizumab, and it has been previously related to this syndrome.21 Another case also reported the development of RPLS in a patient receiving chemotherapy with pemetrexed and cisplatin, but the authors hypothesized that it was not related to pemetrexed but rather to the extended use of dexamethasone and cisplatin.22 Cases of recurrent RPLS have been previously reported and include acute blood pressure elevations as a common finding.9,18,23
Conclusion
In this report we describe a case of a patient with NSCLC who presented with clinical features and neuroimaging findings suggestive of RPLS after the infusion of bevacizumab. Although resolution of most of the clinical symptoms was observed within days, a repeat episode occurred 14 months later. The presence of hypertensive crisis and worsening of proteinuria on the first presentation suggests anti-VEGF–mediated endothelial dysfunction as the mechanism for the development of RPLS. Strict blood pressure control appears to be essential for the prevention of this syndrome, but sudden elevations may be difficult to anticipate. Further research for understanding the mechanism for bevacizumab’s neurologic toxicity and potential prevention measures for its development and recurrence is warranted.
Author Disclosure Statement
Drs Pinto, Kernan, Hoffman, Ibrahim, and Bastos have reported no actual or potential conflicts of interest.
References
- Avastin (bevacizumab) [package insert]. San Francisco, CA: Genentech, Inc; 2010.
- Shord SS, Bressler LR, Tierney LA, et al. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999-1013.
- Marinella MA, Markert RJ. Reversible posterior leucoencephalopathy syndrome associated with anticancer drugs. Int Med J. 2009;39:826-834.
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep. 2008;10:86-91.
- Gressett SM, Shah SR. Intricacies of bevacizumab induced toxicities and their management. Ann Pharmacother. 2009;43:490-501.
- Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000;21: 1199-1206.
- Schwartz RB. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:1743.
- Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205-210.
- Vuorela P, Helske S, Hornig C, et al. Amniotic fluid—soluble vascular endo - thelial growth factor receptor-1 in preeclampsia. Obstet Gynecol. 2000;95:353-357.
- Sugimoto H, Hamano Y, Charytan D, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278:12605-12608.
- Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596-604.
- Allen JA, Adlakha A, Bergethon PR. Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch Neurol. 2006;63:1475-1478.
- Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:981-982.
- El Maalouf G, Mitry E, Lacout A, et al. Isolated brainstem involvement in posterior leukoencephalopathy induced by bevacizumab. J Neurol. 2008;255:295-296.
- Peter S, Hausmann N, Schuster A, Bohem HF. Reversible posterior leuko - encephalopathy syndrome and intravenous bevacizumab. Clin Experiment Ophthalmol. 2008;36:94-96.
- Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980-981.
- Tam CS, Galanos J, Seymour JF, et al. Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies. Am J Hematol. 2004;77:72-76.
- Koopman M, Muller EW, Punt CJ. Reversible posterior leukoencephalopathy syndrome caused by bevacizumab: report of a case. Dis Colon Rectum. 2008;51: 1425-1426.
- Baytan B, Ozdemir O, Demirkaya M, et al. Reversible posterior leukoencephalopathy induced by cancer chemotherapy. Pediatr Neurol. 2010;43:197-201.
- Martin G, Bellido L, Cruz JJ. Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J Clin Oncol. 2007;25:3559.
- Nguyen MT, Virk IY, Villano JL. Extended use of dexamethasone-associated posterior reversible encephalopathy syndrome with cisplatin-based chemotherapy. J Clin Neurosci. 2009;16:1688-1690.
- Hagemann G, Ugur T, Witte OW, Fitzek C. Recurrent posterior reversible encephalopathy syndrome (PRES). J Hum Hypertens. 2004;18:287-289.