Childhood leukemia is the most common form of pediatric cancer in the United States, accounting for nearly 1 of 3 cancers, with an incidence rate of 4.8 per 100,000 persons based on data from 2016 to 2020.1,2 The majority of childhood leukemia cases are acute lymphoblastic leukemia (ALL), which accounts for nearly 75% of cases, whereas acute myeloid leukemia (AML) primarily accounts for the remaining 25% of cases.2 In addition, on average, approximately 750 pediatric patients undergo allogeneic hematopoietic stem cell transplant (HSCT) and nearly 500 patients undergo autologous HSCT each year.3 Patients receiving intensive chemotherapy for AML or ALL and those undergoing HSCT have an increased risk for serious bacterial infections.4-6 Microbiologically defined infections occur in more than 60% of pediatric patients with AML, resulting in a mortality rate of 11%.4 The most common sites of infection include the blood, lungs, and gastrointestinal tract.4,5 Up to 65% of pediatric patients with ALL have an infection resulting from gram-positive and gram-negative organisms, with an incidence rate of infection-related mortality of 2.4%.7,8 For pediatric patients undergoing HSCT, the incidence of bacteremia is between 20% and 44%, with a mortality rate of up to 50% during the posttransplant period.6 One of the most common risk factors for infection across the entire patient population is neutropenia.4-8
To prevent infections, prophylactic antibiotics are often used during periods of neutropenia in pediatric patients with AML, relapsed ALL, and those undergoing autologous or allogeneic HSCT. The use of prophylactic agents is based on the current Infectious Diseases Society of America (IDSA) guidance specific to pediatric cancer and HSCT.9 IDSA recommends the consideration of systemic antibacterial prophylaxis in children with AML and relapsed ALL if they are receiving intensive chemotherapy that is expected to result in severe neutropenia, which is defined as an absolute neutrophil count (ANC) of <500 cells/μL for at least 7 days. For patients undergoing HSCT, IDSA recommends against the routine use of prophylactic antibiotics; however, considerations for use can be made in patients with preceding AML or relapsed ALL in the setting of prolonged neutropenia that may occur during the transplant process. If the decision is made to initiate prophylactic antibiotics, IDSA strongly recommends levofloxacin over other fluoroquinolones because it has adequate coverage of viridans streptococci and Pseudomonas species. Ciprofloxacin may be used as an alternative treatment, but it lacks activity against some gram-positive bacteria, including viridans streptococci.9
Studies have compared treatment with levofloxacin with no prophylaxis or with other antibacterial treatment options.10-15 Some small studies that compared the use of levofloxacin with no prophylaxis in pediatric patients with AML, relapsed ALL or newly diagnosed ALL, or those undergoing autologous HSCT showed that the use of levofloxacin significantly reduced bacteremia and delayed the onset of fever.10-12 In addition, the Children’s Oncology Group conducted a large study that compared the use of prophylactic levofloxacin with no prophylaxis in patients with AML, relapsed ALL, or those undergoing HSCT.13 The study showed a significant decline in the incidence of bacteremia in the group with leukemia; however, for patients undergoing HSCT, no difference in the incidence of bacteremia was found.13 Additional studies compared levofloxacin prophylaxis with other clinician-directed prophylaxis, including cefepime or ciprofloxacin, in patients with AML, newly diagnosed ALL, or those undergoing HSCT.14,15 When compared with clinician-directed prophylaxis, treatment with levofloxacin did not result in a significant difference in the incidence of bacteremia; however, compared with no prophylaxis in patients with newly diagnosed ALL, the use of levofloxacin resulted in a lower incidence of bacteremia.14,15
A secondary outcome for some of these studies included the presence of Clostridiodes difficile infection.10,12-15 The results have varied as to whether levofloxacin reduces or increases the incidence of C difficile compared with other prophylactic agents and no prophylaxis. In addition, with the increased use of levofloxacin, there is a concern for fluoroquinolone resistance. Studies of pediatric patients have reported limited or no data on new fluoroquinolone resistance, with most results showing no significant increases in resistant organisms after patients have received levofloxacin prophylaxis.13-15 However, 1 study of pediatric patients who received levofloxacin prophylaxis for AML or relapsed ALL showed a significant increase in the incidence of gram-negative rod bacteremia that was resistant to levofloxacin after the implementation of a prophylaxis guideline.10
In a study of adults who had autologous HSCT, 16.2% of patients had viridans streptococci bacteremia, with all isolates displaying decreased susceptibility to levofloxacin.16 In addition, the results of a pediatric study that assessed resistant bloodstream infections in patients receiving chemotherapy or undergoing HSCT showed an increasing resistance to ciprofloxacin, with 25.5% of gram-negative blood cultures displaying resistance to ciprofloxacin.17 The risk for resistance to ciprofloxacin increased by approximately 2-fold after previous exposure to a fluoroquinolone, indicating a potential risk for resistance in patients receiving levofloxacin.17
As of September 2018, our institution implemented the use of levofloxacin as the standard prophylactic antibiotic in pediatric patients with AML, relapsed ALL, or those undergoing HSCT. Levofloxacin was selected based on recommendations by IDSA and on the results of the Children’s Oncology Group study.9,13 Levofloxacin is used for the treatment of common pathogenic organisms in this patient population, including Pseudomonas aeruginosa and Streptococcus species.4,9 The objective of this study was to evaluate the rate of bacteremia and the presence of fluoroquinolone resistance in pediatric patients with AML, relapsed ALL, or those undergoing HSCT who received levofloxacin prophylaxis compared with no prophylaxis.
Methods
This single-center, retrospective cohort study included patients who were admitted to Monroe Carell Jr. Children’s Hospital at Vanderbilt in Nashville, TN, to receive chemotherapy or undergo HSCT from November 1, 2017, to July 31, 2020, for the levofloxacin group and from January 1, 2014, to January 30, 2018, for the control group. Overlap of the 2 groups resulted from the gradual adoption of levofloxacin as the standard antibiotic prophylactic agent across providers at the institution.
International Classification of Diseases (ICD)-9 and ICD-10 codes were used to identify the patients who were diagnosed with AML, relapsed ALL, or were undergoing autologous or allogeneic HSCT. In addition, patients were included in the study if they had any period of neutropenia during their hospitalization, which was defined as an ANC of <500 cells/μL. No minimum duration of neutropenia was required. Patients were excluded from chart review if they required treatment antibiotics within 1 day of admission for febrile neutropenia or suspected infection. This study was approved by the Vanderbilt University Institutional Review Board.
The patients who were included in the levofloxacin group could receive medication by oral or intravenous route. Patients aged <5 years received levofloxacin 10 mg/kg per dose every 12 hours, whereas patients aged ≥5 years received 10 mg/kg per dose once daily with a maximum dose of 750 mg. The current standard practice at our institution for patients with AML is to initiate antibiotic prophylaxis for all treatment cycles once their ANC is <500 cells/μL and to discontinue antibiotic prophylaxis after neutrophil recovery.
For patients with relapsed ALL, prophylaxis is typically initiated on admission and is discontinued after neutrophil recovery and the obtainment of minimal residual disease–negative status, unless required by any study protocol. For patients who have had HSCT, prophylaxis is typically initiated on day 1 after the transplant, or earlier in the setting of prolonged neutropenia, and is discontinued after neutrophil recovery unless the patient is diagnosed with acute graft-versus-host disease of the gut or is receiving prednisone doses of ≥1 mg/kg daily. The control group received no antibiotic prophylaxis, despite meeting the criteria for study initiation.
For the study’s purposes, the neutropenic period was defined in the levofloxacin group as starting on day 1 of prophylaxis and ending once the ANC recovered to >500 cells/μL. In the control group, the neutropenic period was defined as the time in which ANC remained <500 cells/μL.
The primary end point of the study was the incidence of bacteremia in patients receiving levofloxacin prophylaxis compared with the control, which was defined as a positive blood culture with an identified pathogenic organism at any time during the admission. A subgroup analysis was performed to assess the incidence of breakthrough bacteremia, which was defined as a positive blood culture within 2 days of transition to empiric treatment antibiotics, with cefepime being the most frequently used empiric antibiotic.
The secondary end points included the presence of C difficile infection, the incidence of clinically documented infection, and the occurrence of fluoroquinolone resistance as determined by phenotypic testing within 6 months of receiving levofloxacin prophylaxis compared with the control.
One-step and 2-step C difficile tests were used during the study period, because the adoption of the 2-step test occurred in 2019. For the 2-step test, the patients were considered positive if they had a positive polymerase chain reaction (PCR) test and a toxin result, whereas the 1-step test only required a positive PCR test result. Clinically documented infection was defined as any positive culture with an identified pathogenic organism at any time from the patient’s first admission to 6 months after their final discharge if they were admitted for multiple encounters during the study period.
The baseline demographics that were collected included patient age, sex, underlying diagnosis, type of access, and days the ANC was <500 cells/μL. The type of access was divided between the patients with a port-a-cath, external central venous line (CVL), and peripherally inserted central catheter. The external CVL could be a Hickman, Broviac, or Trifusion catheter. Patients may have had multiple types of access during the study period.
IBM SPSS Statistics version 26 (IBM Corporation) was used for all statistical analyses that were conducted. Descriptive statistics were calculated for the observational outcomes. Percentages were reported for the categorical data whereas the median, interquartile range, and range were reported for the continuous end points. For the primary and secondary outcomes, statistical significance was calculated using an alpha of 0.05. P values were calculated using chi-square tests for expected frequencies of >5 and Fisher exact test for expected frequencies of ≤5.
Results
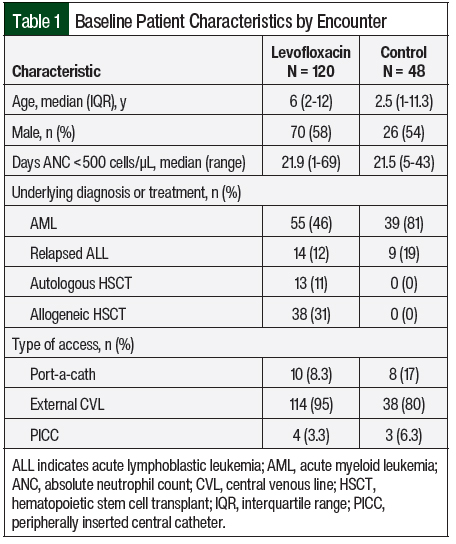
A total of 168 patient encounters were included in the study, of which 120 were in the levofloxacin group and 48 were in the control group. The baseline characteristics were similar between both groups, except for the underlying diagnosis or treatment (Table 1). The levofloxacin group was composed of 46% patients with AML, 12% with relapsed ALL, 11% who had autologous HSCT, and 31% who had allogeneic HSCT. In comparison, the control group was composed of 81% of patients with AML and 19% with relapsed ALL with no patients who had autologous or allogeneic HSCT. Most patients in both groups had an external CVL; however, more patients in the control group than in the levofloxacin group had a port-a-cath (17% vs 8.3%, respectively).
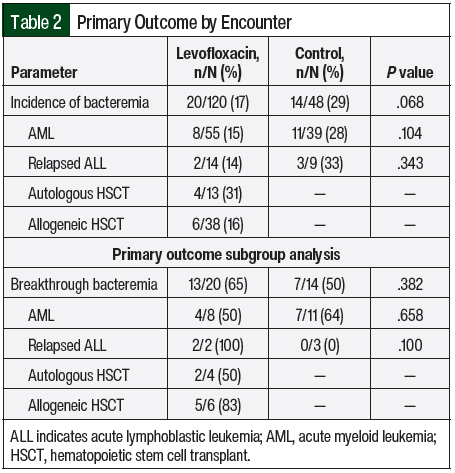
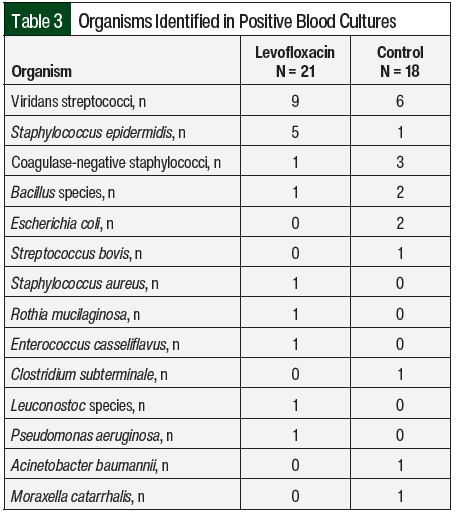
The results of the primary outcome are shown in Table 2. Bacteremia occurred in 17% of the patients who received levofloxacin and in 29% of the control patients (P=.068). The organisms that were identified from these positive blood cultures are shown in Table 3, with more than 1 organism isolated in a small number of blood cultures. The incidence of bacteremia was then divided into subgroups based on the patients’ diagnosis of AML or relapsed ALL. Of the patients with AML, 15% in the levofloxacin group had bacteremia compared with 28% in the control group; in the patients with relapsed ALL, 14% in the levofloxacin group had bacteremia compared with 33% of patients in the control group (Table 2).
For the levofloxacin and control groups, each incidence of bacteremia was evaluated for consideration as breakthrough bacteremia (Table 2). Of the total cases of bacteremia evaluated, 65% in the levofloxacin group and 50% in the control group were considered breakthrough (P=.382). The results were also broken down into patient diagnosis of AML or relapsed ALL. In patients with AML, 50% of the bacteremias in the levofloxacin group and 64% in the control group were considered breakthrough; in patients with relapsed ALL, 100% of the bacteremias in the levofloxacin group and none in the control group were considered breakthrough.
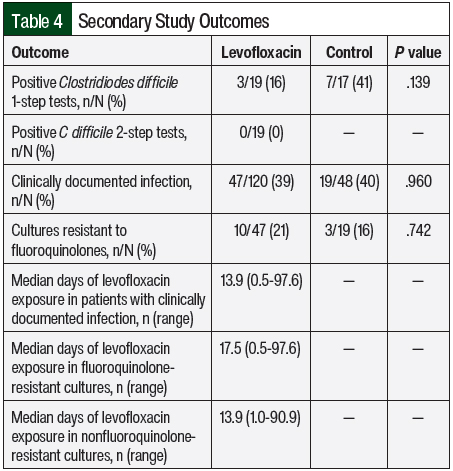
The secondary study outcomes and results are shown in Table 4. Of the 1-step tests performed, 3 of 19 (16%) were positive for C difficile in the levofloxacin group and 7 of 17 (41%) were positive for C difficile in the control group (P=.139). Two-step tests were only performed in the levofloxacin group, and none were positive for C difficile. Clinically documented infections occurred in 39% of the patients who received levofloxacin and in 40% of the control group (P=.96). Of these documented infections, 10 of 47 (21%) cultures in the levofloxacin group and 3 of 19 (16%) cultures in the control group were resistant to fluoroquinolones (P=.742).
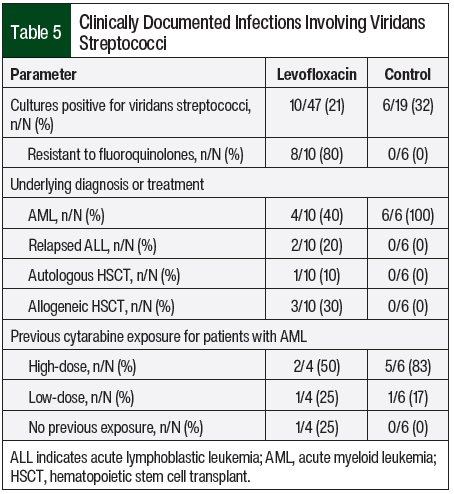
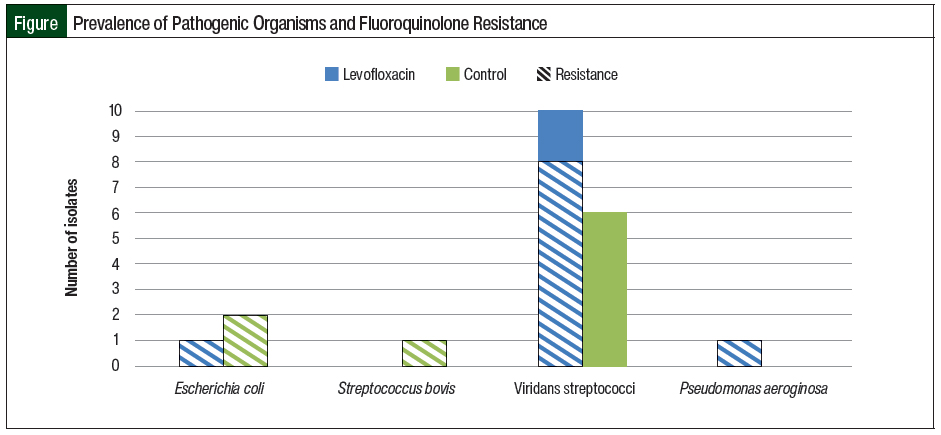
A total of 10 clinically documented infections in the levofloxacin group had cultures that were positive for viridans streptococci (Table 5, Figure). Of these 10 cultures, 8 were resistant to levofloxacin. In comparison, 6 cultures that were positive for viridans streptococci were identified in the control group, none of which were resistant to fluoroquinolones. Additional characteristics of patients with cultures that were positive for viridans streptococci are shown in Table 5.
After the use of levofloxacin prophylaxis, changes in the distribution of underlying diagnosis and previous cytarabine exposure were observed (Table 5). In addition, 1 culture that was positive for Escherichia coli and 1 culture that was positive for P aeruginosa were identified in the levofloxacin group, both of which were resistant to ciprofloxacin and levofloxacin. Two cultures that were positive for E coli were identified in the control group, both of which were resistant to fluoroquinolones, despite a lack of levofloxacin use for prophylaxis (Figure).
Discussion
The results of this single-center, retrospective cohort study showed that the use of levofloxacin prophylaxis did not significantly reduce the incidence of bacteremia compared with no prophylaxis; however, the results showed a trend toward reduction in that 17% of patients who received levofloxacin had bacteremia compared with 29% of the control patients. This trend was also seen in the subgroup analysis of the incidence of bacteremia based on patient diagnosis, despite the results not meeting statistical significance. The results for the primary outcome may have reached statistical significance had a larger sample size been obtained as in previous studies that compared the use of levofloxacin prophylaxis with no prophylaxis.10-13,15
Interestingly, when evaluated for consideration as breakthrough bacteremia, a greater percentage of bacteremias in the levofloxacin group were deemed to be breakthrough than in the control group. Although not statistically significant, the question then arises whether levofloxacin provided adequate and immediate prophylaxis against organisms in the bloodstream. However, the results were likely influenced by the overall low number of total bacteremias, because the levofloxacin group had 20 incidences of bacteremia and the control group had 14 incidences of bacteremia to evaluate.
No significant differences were found between the levofloxacin and control groups for all of the secondary outcomes. There was a clinically significant decrease in positive 1-step C difficile tests in the levofloxacin group compared with the control group. Of the 3 positive 1-step tests in the levofloxacin group, 2 may have been related to levofloxacin exposure. One patient received 56 days of levofloxacin treatment followed by 16 days of empiric antibiotic treatment before testing positive for C difficile, whereas another patient received 28 days of treatment with levofloxacin followed by 8 days of empiric antibiotic treatment before testing positive for C difficile. The third patient received a single dose of levofloxacin before testing positive for C difficile with no previous exposure to levofloxacin for more than 1 month.
The 1-step test provides only limited information because it does not differentiate between toxigenic and nontoxigenic strains of C difficile. The 2-step test, which confirms the presence of a toxigenic strain of C difficile, was not implemented until after the adoption of levofloxacin as the prophylactic agent. A comparison of positive 2-step tests between the groups would have provided more accurate information on the incidence of true C difficile infection.
For the secondary outcome of clinically documented infection, a similar incidence was found between the groups, with nearly 20% of the cultures being resistant to fluoroquinolones. Despite not reaching statistical significance, the results were clinically significant, especially for cultures with viridans streptococci isolates; 8 of 10 cultures were resistant to levofloxacin after the use of prophylaxis compared with none of the 6 cultures in the control group that received no prophylaxis.
In 2018, 2019, and 2020, our institution reported fluoroquinolone susceptibility rates of 87%, 92%, and 94%, respectively, for viridans streptococci, showing that overall resistance to fluoroquinolones was low. In addition, the only culture with a P aeruginosa isolate in the levofloxacin group was also resistant to fluoroquinolones. Although there were no isolates in the control group, from 2015 to 2020, our institution had an average P aeruginosa susceptibility of 91% to fluoroquinolones, with a decrease in susceptibility from 97% in 2019 to 87% in 2020. Despite an overall low number of cultures evaluated for resistance to fluoroquinolones, the results add to the current literature showing an increased risk for resistance after fluoroquinolone exposure.10,16,17
Several study confounders were identified that could have impacted the study results. First, the implementation of the 2-step C difficile test occurred mid-study in 2019, and this was accounted for by separating the results based on whether a 1-step or 2-step test was conducted. However, because the 2-step test was implemented after the data collection period for the control group, no comparison could be made between the groups. Since the implementation of the 2-step test, our institution treats C difficile only if the PCR test and the toxin result are positive. Before the implementation of the 2-step test, a positive 1-step test could have led to treatment based on symptoms alone, which could have been affected by a variety of factors in this population. A comparison between the groups for the 2-step test would have been useful to determine if the use of levofloxacin changed the incidence of infection with toxigenic strains requiring treatment, because the current results only show a trend toward a reduction in infection or colonization with the 1-step test.
A second confounder involved the change in practice surrounding the implementation of chlorhexidine baths mid-study in 2018. Most patients had either an external CVL or a port, which could have led to the introduction of bacteria from the skin. Because of this, practice changes involving the use of chlorhexidine could have affected the incidence of bacteremia and clinically documented infections.
A third study confounder was the practice of discharging patients from the study institution with a recovering ANC of <500 cells/μL. Per data collection methods, these patients were considered to have neutropenia until they had a confirmed ANC of >500 cells/μL at a follow-up clinic evaluation or at the next hospital admission, which could have been several days to weeks after discharge. This would have extended the period in which the patient was considered to have neutropenia and to be at risk for bacteremia and clinically documented infection.
In addition to the discussed confounders, the gradual adoption of levofloxacin prophylaxis at our institution could have potentially introduced bias because the patients who received levofloxacin prophylaxis early in the study period may have had a higher risk for infection compared with the control group during the overlapping time period.
Limitations
This study has several limitations. This was a small, single-institution study, which limits its applicability to other institutions that may have different infection prevention protocols as well as different baseline antibiotic resistance patterns from ours. In addition, there was an insufficient number of patients in the study to detect significant differences, and the outcomes may have varied if more patients were included.
Levofloxacin prophylaxis was implemented in all patients undergoing HSCT throughout the study period, which did not allow for a control group in this patient population. If a practice change had occurred during the study period, an additional subgroup analysis of these patients could have been performed, such as that conducted in the Children’s Oncology Group study.13 There was also a change in electronic health record software mid-study in 2017 that impacted the data collection processes and the ability to find information for review. Last, additional adverse events related to the use of fluoroquinolones beyond those addressed in the study were not assessed.
Conclusion
The use of levofloxacin prophylaxis did not result in a significant reduction in the incidence of bacteremia compared with the control in patients with AML, relapsed ALL, or in those undergoing autologous or allogeneic HSCT. However, the study results showed a potential trend toward a reduction in the incidence of bacteremia without significantly increasing the incidence of C difficile infection.
With a high risk for infection-related mortality, prophylaxis should still be strongly considered. However, as the use of levofloxacin increases, fluoroquinolone resistance may continue to rise and considerations of an alternative antibiotic treatment for prophylaxis may be necessary. Larger studies should be conducted to continue to assess the ongoing risk for resistance.
References
- National Cancer Institute SEER program. Cancer stat facts: childhood leukemia (ages 0-19). https://seer.cancer.gov/statfacts/html/childleuk.html. Accessed May 30, 2023.
- American Cancer Society. About childhood leukemia. Revised February 12, 2019. www.cancer.org/content/dam/CRC/PDF/Public/8693.00.pdf. Accessed May 30, 2022.
- Khandelwal P, Millard HR, Thiel E, et al. Hematopoietic stem cell transplantation activity in pediatric cancer between 2008 and 2014 in the United States: a Center for International Blood and Marrow Transplant Research report. Biol Blood Marrow Transplant. 2017;23:1342-1349.
- Sung L, Lange BJ, Gerbing RB, et al. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532-3539.
- Bochennek K, Hassler A, Perner C, et al. Infectious complications in children with acute myeloid leukemia: decreased mortality in multicenter trial AML-BFM 2004. Blood Cancer J. 2016;6:e382.
- Vaz de Macedo A. Comment on: Bacteremia in pediatric patients with hematopoietic stem transplantation. Hematol Transfus Cell Ther. 2020;42:1-4.
- Kuo FC, Wang SM, Shen CF, et al. Bloodstream infections in pediatric patients with acute leukemia: emphasis on gram-negative bacteria infections. J Microbiol Immunol Infect. 2017;50:507-513.
- O’Connor D, Bate J, Wade R, et al. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood. 2014;124:1056-1061.
- Lehrnbecher T, Fisher BT, Phillips B, et al. Guideline for antibacterial prophylaxis administration in pediatric cancer and hematopoietic stem cell transplantation. Clin Infect Dis. 2020;71:226-236.
- Davis A, Stevens AM, Brackett J, et al. Levofloxacin prophylaxis for pediatric leukemia patients: longitudinal follow-up for impact on health care-associated infections. Pediatr Blood Cancer. 2022;69:e29525.
- Hafez HA, Yousif D, Abbassi M, et al. Prophylactic levofloxacin in pediatric neutropenic patients during autologous hematopoietic stem cell transplantation. Clin Transplant. 2015;29:1112-1118.
- Sulis ML, Blonquist TM, Stevenson KE, et al. Effectiveness of antibacterial prophylaxis during induction chemotherapy in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65:e26952.
- Alexander S, Fisher BT, Gaur AH, et al; for the Children’s Oncology Group. Effect of levofloxacin prophylaxis on bacteremia in children with acute leukemia or undergoing hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2018;320:995-1004. Erratum in: JAMA. 2019;322:84.
- Patel B, Noda A, Godbout E, et al. Levofloxacin for antibacterial prophylaxis in pediatric patients with acute myeloid leukemia or undergoing hematopoietic stem cell transplantation. J Pediatr Pharmacol Ther. 2020;25:629-635.
- Wolf J, Tang L, Flynn PM, et al. Levofloxacin prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. Clin Infect Dis. 2017;65:1790-1798.
- Razonable RR, Litzow MR, Khaliq Y, et al. Bacteremia due to viridans group streptococci with diminished susceptibility to levofloxacin among neutropenic patients receiving levofloxacin prophylaxis. Clin Infect Dis. 2002;34:1469-1474.
- Castagnola E, Bagnasco F, Mesini A, et al. Antibiotic resistant bloodstream infections in pediatric patients receiving chemotherapy or hematopoietic stem cell transplant: factors associated with development of resistance, intensive care admission and mortality. Antibiotics (Basel). 2021;10:266.