Ovarian cancer ranks among the top 5 causes of death in women and is the leading cause of death from gynecologic cancer in the United States.1 Since the early 2000s, few therapeutic advances had been made until the accelerated FDA approval of olaparib in 2014. Olaparib, the first poly (ADP-ribose) polymerase (PARP) inhibitor approved, was initially indicated for monotherapy in patients with ovarian cancer and germline BRCA mutations after ≥3 previous lines of chemotherapy.2 Since 2014, olaparib, niraparib, and rucaparib have gained approvals beyond monotherapy, most notably in the first-line and subsequent-line maintenance setting.3-7 After the publication of the SOLO-1 trial, which showed a substantial progression-free survival (PFS) benefit in patients with a BRCA1 or BRCA2 mutation,3 olaparib was the first PARP inhibitor approved by the FDA in the first-line maintenance setting for patients with somatic or germline BRCA mutations. Based on the phase 3 PRIMA trial,4 niraparib was later approved for use in the first-line maintenance setting regardless of homologous recombination deficiency (HRD) status. Niraparib, olaparib, and rucaparib are now all approved by the FDA in the recurrent maintenance setting, regardless of HRD, based on the NOVA, SOLO-2, and ARIEL-3 trials, respectively.5-7
Before reviewing the mechanism of PARP inhibitors, it is important to examine the role of PARP proteins. DNA breaks are repaired by various mechanisms to retain normal cellular function and growth.8 Single- and double-stranded DNA breaks are repaired by different mechanisms. One mechanism used to repair single-stranded DNA breaks is base excision repair, and the family of PARP proteins plays an essential role in this repair mechanism.8 PARP inhibitors target these proteins, inhibiting their ability to bind and initiate the repair process leading to an accumulation of single-stranded DNA breaks.8 The accumulation of single-stranded DNA breaks can result in double-stranded DNA breaks, which would normally be restored via homologous recombination repair (HRR).9
HRR is a complex process that includes many proteins and genes, most notably BRCA1 and BRCA2.9 When harboring a mutation in BRCA1, BRCA2, or other HRR genes, cells are considered to have HRD and must use an alternative repair mechanism to fix double-stranded DNA breaks.10 One alternative mechanism, nonhomologous end joining, is prone to errors because it does not use a homologous template, but it can minimize the accumulation of DNA damage and apoptosis.8 However, by pairing HRD with a PARP inhibitor, a concept referred to as synthetic lethality is exploited, and the impairment of multiple DNA repair mechanisms leads to more certain cell death.8-10
Germline and somatic mutations are 2 different types of HRR mutations, which both lead to HRD.10 The key differences between the mutations include the inheritance pattern, where the mutations are found, and how they change over time. Germline mutations occur in germ cells and are inheritable, whereas somatic mutations occur in the tumor microenvironment and are not inheritable.10 Approximately 41% to 50% of ovarian carcinomas are estimated to exhibit HRD.8 The most common germline mutations include BRCA1, BRCA2, RAD51C, and CHEK1, whereas the most common somatic mutations include BRCA1, BRCA2, EMSY, PTEN, and FANC.8
PARP inhibitors can be used in BRCA wild-type cancer or patients with no known HRD, but there are greater PFS benefits in patients with HRD.4,5,7 For example, patients with HRD who received niraparib in the PRIMA trial had a median PFS of 21.9 months versus 13.8 months in patients for whom HRD status was not determined.4 However, the trial did not differentiate between germline or somatic HRD.4 In fact, clinical trials of PARP inhibitors have included very few patients with somatic mutations.3,6,7 In the SOLO-1 trial, only 2 of the 391 patients harbored a somatic BRCA1 or BRCA2 mutation.3 The SOLO-2 trial allowed patients with germline and somatic HRD; however, all patients who were randomized harbored a germline BRCA1 or BRCA2 mutation.6 In ARIEL-3, 56 of 196 patients with BRCA mutation had somatic mutations.7 Similar to the PRIMA trial, the NOVA trial did not differentiate whether HRD resulted from germline or somatic mutations.4,5 Therefore, we have limited data directly comparing the duration of response with maintenance PARP inhibitors for patients with somatic or germline HRD. Defining this response could provide clarity for practitioners regarding the expected duration of response to PARP inhibitor maintenance therapy based on the type of mutation harbored and for patients regarding information on which to base expectations.
In this retrospective study, we aimed to find if germline or somatic HRD impacted the duration of response to PARP inhibitors used for the maintenance treatment of ovarian cancer, regardless of the line of therapy.
Methods
A single-center, retrospective chart review was conducted for patients who received a PARP inhibitor for first-line or subsequent-line maintenance treatment from May 1, 2017, to September 1, 2020. Patients were aged ≥18 years with ovarian, fallopian tube, or primary peritoneal cancer who received follow-up care at The University of Kansas Health System. Patients were excluded if they received bevacizumab in combination with PARP inhibitor maintenance therapy, were enrolled in a clinical trial, or received PARP inhibitors as treatment rather than maintenance therapy.
The following information was collected for each patient: age, cancer diagnosis, stage at diagnosis, neoadjuvant chemotherapy, PARP inhibitor maintenance line of therapy (first-line vs subsequent-line), presence or absence of germline or somatic mutations in BRCA1 or BRCA2 or other HRR genes, duration of PARP inhibitor response, and day 1 of subsequent chemotherapy if the patient had relapsed disease. The patients had previously undergone tumor molecular testing or next-generation sequencing to determine their HRD status. The patients were considered to have HRD if they exhibited mutations in any of the following genes: BRCA1, BRCA2, EMSY, PTEN, RAD51C, RAD51D, RAD50, ATM/ATR, FANC, BARD1, BRIP1, CHEK1, CHEK2, FAM175A, NBN, PALB2, MRE11A, MMR, and TP53.8 Variants of unknown significance were not considered HRD.
Definition of Clinical End Points
The primary end point of time to next treatment (TTNT) was defined as the time from PARP inhibitor initiation to day 1 of subsequent chemotherapy and was compared between 3 patient cohorts, including the presence of germline HRD, the presence of somatic HRD, or no known HRD. The event of interest occurs when a patient starts the subsequent treatment. TTNT is a helpful measure of the duration of clinical benefit and has advantages over standard end points for our study. TTNT and PFS have similar interpretation, but the event of PFS is disease progression. Because clinician-assessed disease progression is more difficult to abstract during chart review, the surrogate marker of TTNT was chosen to measure clinical benefit. In a comparison of real-world time-to-event end points by Walker and colleagues, TTNT estimates were an effective substitute for PFS estimates, having comparable or greater than real-world PFS estimates while allowing researchers the use of an end point that does not require exhaustive chart review.11
Statistical Analysis
TTNT allows for easy measurement of the period of therapeutic benefit by measuring the interval between the initiation of one treatment to the initiation of the next therapy. TTNT can assess the duration of clinical benefit provided to those receiving PARP inhibitors without requiring a date of disease progression, which is required when assessing PFS.12
Cox proportional hazard models were used to test the association between TTNT and HRD mutation and were fit using the Cox proportional hazard function in the survival R package. A subsequent Cox proportional hazard model was fit adjusting for PARP inhibitor line of therapy (first-line or subsequent-line). An interaction term was added to a final model to assess if the line of therapy differs within each mutation group. Grambsch-Therneau tests indicated no violation of the proportional hazard assumption.13 Because very few patients had the event (n=28; 37.8%), restricted mean TTNT was used to represent the average event-free time up to 25 months, which was the latest follow-up time recorded among the cohorts.14 All statistical analyses were conducted using the R statistical programming language software, version 4.0.2 (R Foundation for Statistical Computing; Vienna, Austria).
Results
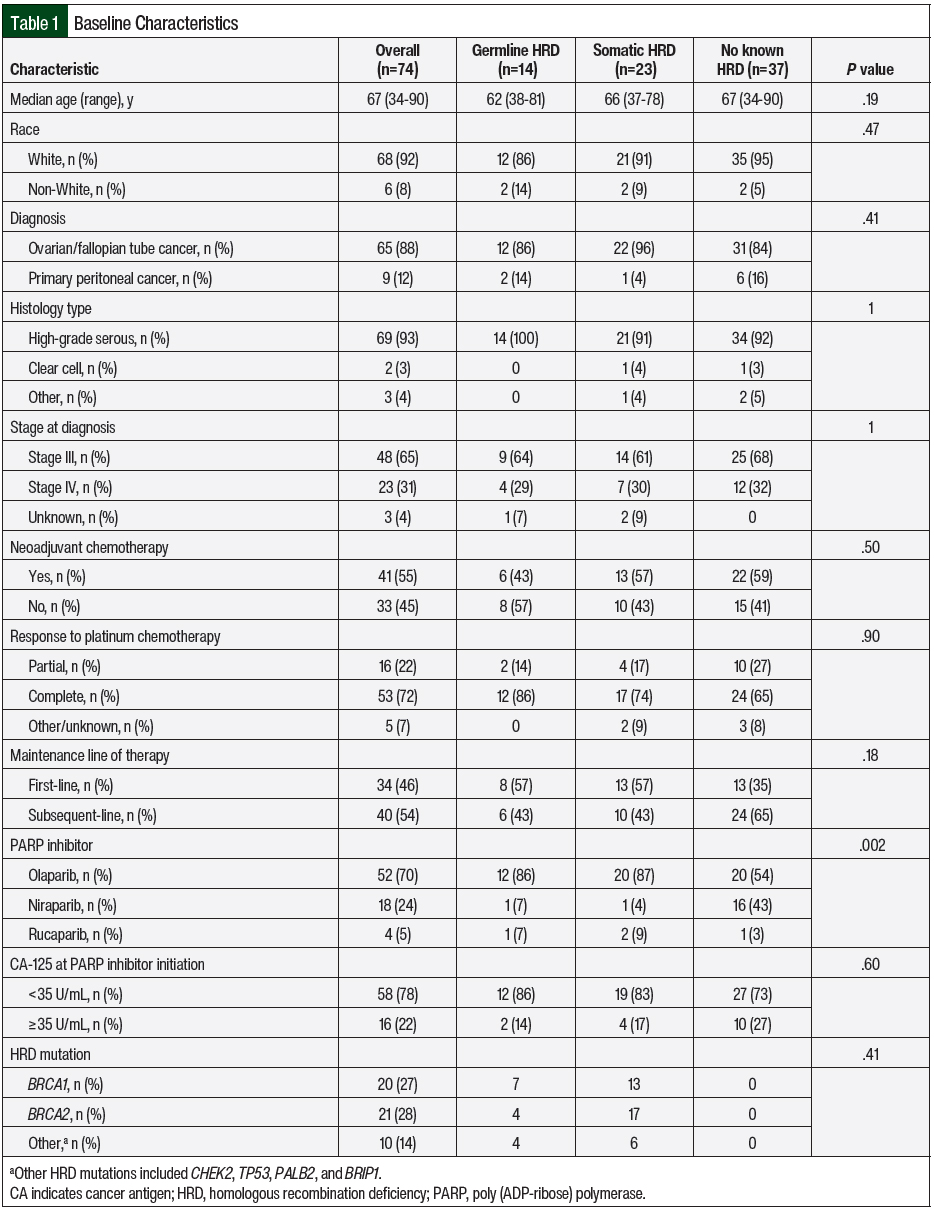
Of 139 charts reviewed, 74 patients met our eligibility criteria. Germline and somatic HRD were identified in 14 and 23 patients, respectively. The most common HRD mutations in the germline HRD and somatic HRD cohorts were BRCA1 (n=20; 27%) and BRCA2 (n=21; 28%). Other germline mutations included CHEK2, TP53, and BRIP1. The other somatic mutations that were identified were TP53 and PALB2. A total of 2 patients had germline and somatic HRD. For this study, we assumed that germline HRD takes precedence over somatic HRD based on the known predisposition of inherited cancers with germline mutations; therefore, these patients were included in the germline HRD cohort.10 The remaining 37 patients had no known HRD.
More than half (n=41; 55%) of the patients received neoadjuvant chemotherapy, with carboplatin and paclitaxel being the primary regimen in most patients (n=61; 82%). PARP inhibitor maintenance therapy was used in the first-line setting for 46% of the patients, whereas the other 54% received PARP inhibitors in the subsequent maintenance setting. The most common PARP inhibitor received was olaparib (n=52; 70%), followed by niraparib (n=18; 24%) and rucaparib (n=4; 5%). Additional patient demographics are shown in Table 1. Based on Fisher exact test of independence between the baseline characteristics and the 3 cohorts, the only characteristic that was significantly different was the PARP inhibitor received.
In all, 33 (44.6%) patients had relapsed disease while receiving PARP inhibitor maintenance therapy, and 28 (84.8%) of those patients received subsequent chemotherapy. These 28 patients were considered events in the TTNT analysis. The remaining 5 patients had not yet started subsequent chemotherapy at the time of data collection and were not considered as events in the TTNT analysis.
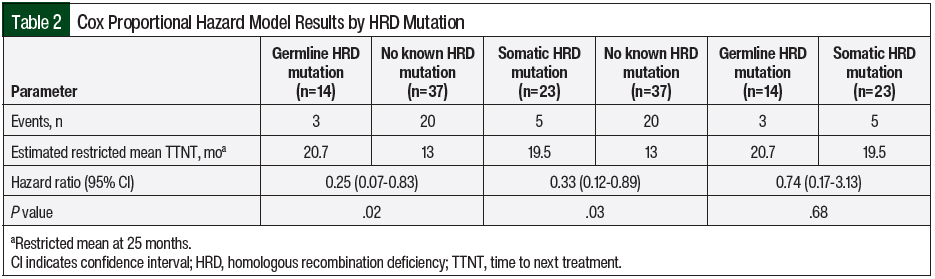
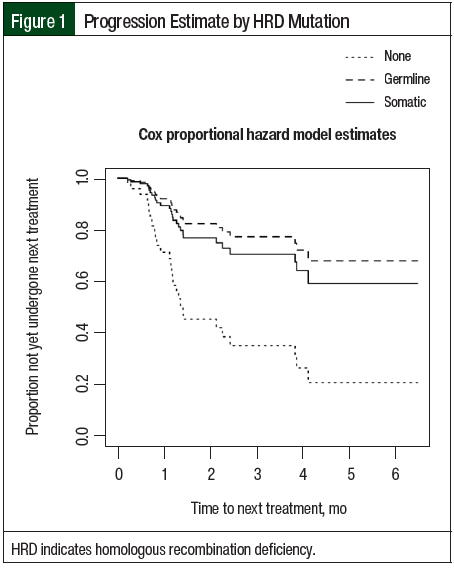
The estimated restricted mean TTNT was 13 months for the patients with no known HRD versus 20.7 months for the patients with germline HRD (no known vs germline, hazard ratio [HR], 0.25; 95% confidence interval [CI], 0.07-0.83; P=.02). Similarly, the estimated restricted mean TTNT was 19.5 months for the patients with somatic HRD (no known vs somatic, HR, 0.33; 95% confidence interval [CI], 0.12-0.89; P=.03). There was no significant difference in TTNT between the patients with germline HRD and the patients with somatic HRD (germline vs somatic, HR, 0.74; 95% CI, 0.17-3.13; P=.68; Table 2 and Figure 1).
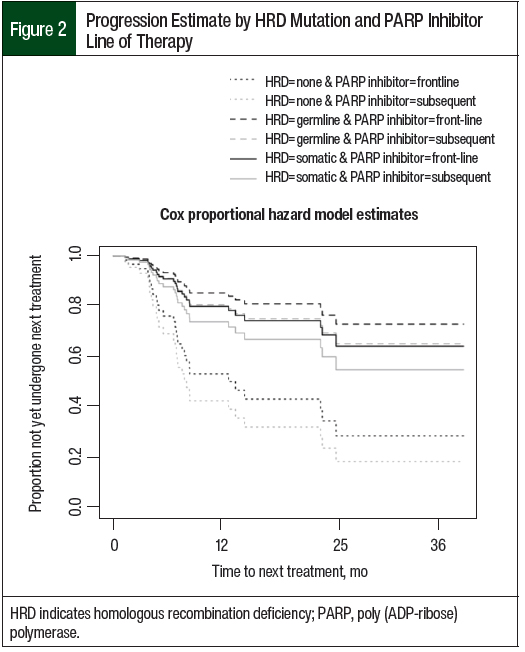
The Cox proportional hazard model was then further adjusted for PARP inhibitor maintenance line of therapy (Figure 2). The mean TTNT remained the same for the 3 cohorts, but the HRs associated with mutation status did change. The patients with no known HRD had a significantly shorter TTNT compared with the patients with germline HRD (no known vs germline, 13 months vs 20.7 months; HR, 0.25; 95% CI, 0.07-0.86; P=.03). Likewise, the patients with no known HRD had a significantly shorter TTNT compared with patients with somatic HRD (no known vs somatic, 13 months vs 19.5 months; HR, 0.35; 95% CI, 0.13-0.96; P=.04). Again, the TTNT was not significantly different between patients with germline HRD and patients with somatic HRD (germline vs somatic, 20.7 months vs 19.5 months; HR, 0.71; 95% CI, 0.17-3.04; P=.65).
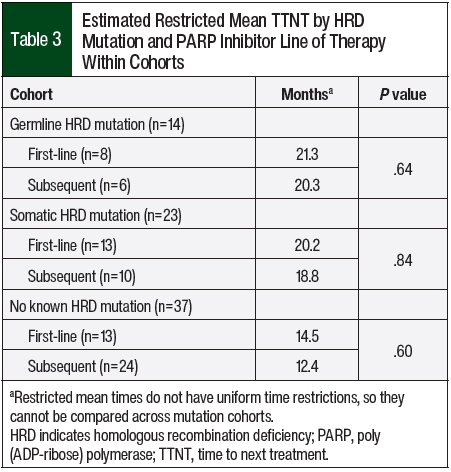
The Cox proportional hazard model was further modified with an interaction term to evaluate any difference in TTNT within the cohorts based on whether a PARP inhibitor was used as first-line maintenance or subsequent-line maintenance therapy (Table 3). Because this model included 6 unique cohorts of mutation and line of therapy, the TTNT differs from previous models. However, the TTNT can be compared only within mutation groups and not across mutation groups, because restricted mean times do not have uniform time restrictions. Within the no known HRD group, the TTNT was not significantly different between the patients who received subsequent-line PARP inhibitor maintenance therapy and those who received first-line PARP inhibitor maintenance therapy (no known subsequent vs first-line, 12.4 months vs 14.5 months; HR, 1.32; 95% CI, 0.48-3.64; P=.60). Likewise, the TTNT was not significantly different between patients in the germline HRD cohort (germline subsequent vs first-line, 20.3 months vs 21.3 months; HR, 1.54; 95% CI, 0.26-9.25; P=.64) and those in the somatic HRD cohort (somatic subsequent vs first-line, 18.8 months vs 20.2 months; HR, 1.29; 95% CI, 0.12-14.40; P=.84).
Safety
A total of 7 (9.5%) patients discontinued PARP inhibitors as a result of adverse events, with hematologic adverse events leading to permanent treatment discontinuation in 4 of the 7 (57.1%) patients. Hematologic adverse events included grade 1 neutropenia (n=1; 14.3%), grade 2 anemia (n=1; 14.3%), and grades 2 and 4 thrombocytopenia (n=2; 28.6%). Other reasons for treatment discontinuation included nausea, jaw pain, headache, hyperkalemia, acid reflux, and worsened renal function. Of the 74 patients evaluated, 1 patient had been diagnosed with acute myeloid leukemia (AML). Olaparib was the most frequently discontinued PARP inhibitor (n=4; 7.7%), followed by niraparib (n=2; 11.1%), and rucaparib (n=1; 25%).
Discussion
Before the approval of PARP inhibitors for maintenance therapy, patients who received treatment for ovarian cancer typically underwent observation after the completion of surgery and first-line chemotherapy. Since 2014, PARP inhibitors have gained several FDA approvals for use in the maintenance setting, including in patients without BRCA mutations or other HRD. Our study is one of the first reports to compare the impact of germline or somatic mutations on response to PARP inhibitor maintenance therapy in ovarian cancer. Our study confirmed that patients with no known HRD had a significantly shorter time to subsequent chemotherapy compared with those with germline or somatic HRD. However, there was no significant difference in the duration of response to PARP inhibitor maintenance therapy if a patient had a germline or somatic mutation. When adjusted for first-line or subsequent-line maintenance treatment, the time until subsequent chemotherapy was administered remained significantly longer among patients with germline and somatic HRD compared with those with no known HRD. When assessing the TTNT for the patients within the same cohort adjusted for first-line or subsequent-line maintenance treatment, the duration of response did not differ significantly in any of the cohorts. The response to PARP inhibitor therapy was preserved regardless of whether it was used in the first-line or subsequent-line maintenance setting. Therefore, we expect the duration of response to PARP inhibitor maintenance therapy to be similar in patients regardless of use in the first-line or subsequent-line maintenance setting. Going forward, based on our study, we anticipate PARP inhibitors will be used less frequently in subsequent-line maintenance treatment because of the expanded indications of PARP inhibitors in the first-line maintenance setting and the lack of current data supporting a retrial of PARP inhibitors once disease progression has occurred while receiving PARP inhibitor therapy.
Among the clinical trials that led to the approval of PARP inhibitors, treatment discontinuation resulting from adverse events ranged from 11% to 13%.4,6,7 These findings are slightly higher than the 9.5% discontinuation rate in our analysis. In the PRIMA, SOLO-2, and ARIEL-3 trials, hematologic adverse events were the most frequent adverse events that led to the discontinuation of PARP inhibitor maintenance therapy and occurred in 10.2%, 35.9%, and 38.2% of patients, respectively.4,6,7 Hematologic adverse events included anemia, thrombocytopenia, neutropenia, and leukopenia. In our study, of the 7 patients who discontinued PARP inhibitor maintenance therapy, 4 (57.1%) patients discontinued therapy as a result of hematologic adverse events. In the SOLO-2 and ARIEL-3 trials, approximately 1% of patients had AML after PARP inhibitor maintenance therapy.6,7 In our study, 1 (1.4%) patient was diagnosed with AML 6 months after discontinuing PARP inhibitor maintenance therapy. The PARP inhibitor had been discontinued as a result of disease progression.
PARP inhibitors are usually well tolerated, and they also offer hope regarding a disease with few therapeutic advances since the early 2000s. Like many new therapies, there are more questions than answers. Our study helps to answer if the duration of response to PARP inhibitor maintenance therapy differs based on whether an HRD mutation is germline or somatic. This is important because we know patients with germline BRCA or other germline HRD mutations respond to maintenance PARP inhibitor therapy, as demonstrated by clinical trials, but now we can confirm patients with somatic HRD mutations have a similar response. As the number of indications expands for PARP inhibitors, understanding the impact of gene mutations on response to therapy can guide clinicians in selecting the appropriate maintenance therapy.
Limitations
This study has limitations. Data collection and accuracy are limited to the completeness and accuracy of the electronic medical record. Because our providers do not use tumor molecular testing that calculates an HRD score, HRD was determined simply by the presence of a mutation in one of the HRR genes.
The PARP inhibitor received by patients with no known HRD was significant when compared with the other cohorts. This finding is not unexpected because niraparib was the first PARP inhibitor to be FDA approved in the maintenance setting regardless of HRD. In contrast, olaparib was used more often in the HRD cohorts compared with the no known HRD cohort because it was the first PARP inhibitor to be FDA approved for patients with BRCA1 or BRCA2 mutations in the first-line maintenance setting.
The small sample size may hinder the statistical power for detecting differences and associations between the groups. For example, in Figure 2, the Cox proportional hazard model estimates show longer TTNT for those who received first-line PARP inhibitor maintenance across all mutation groups. If a difference exists, it may be detected with a larger cohort.
Conclusion
In this single-center, retrospective chart review, patients with germline or somatic HRD receiving PARP inhibitors for maintenance treatment of ovarian cancer had a significantly longer TTNT compared with patients with no known HRD, regardless of use in the first-line or subsequent-line setting. There was no difference in TTNT between patients with germline HRD or somatic HRD. Within the cohorts, the periods of response did not differ between the patients receiving PARP inhibitor maintenance therapy in the first-line and those receiving PARP inhibitor maintenance therapy in the subsequent-line setting. Patients and providers can confidently expect similar periods of response regardless of germline or somatic HRD or maintenance line of therapy.
Funding Source
This study was supported by a grant from the National Cancer Institute.
Author Disclosure Statement
Dr Mahmoudjafari is on the advisory board of GlaxoSmithKline, Bristol-Myers Squibb, Omeros, and Incyte, and is on the Speaker’s Bureau of Omeros; Dr Meier was a postdoctoral researcher at The University of Kansas Medical Center when his work on this project was completed; Ms Shae and Dr Koestler received grant support from the National Cancer Institute; Dr Adolphsen, Dr Monson, Dr Martin, and Dr Bohnenkamp have no conflicts of interest to report.
References
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 2.2023; June 2, 2023. www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed July 10, 2023.
- Kim G, Ison G, McKee AE, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline brca-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21(19):4257-4261.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- González-Martín A, Pothuri B, Vergote I, et al; for the PRIMA/ENGOT-OV26/GOG-3012 investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- Mirza MR, Monk BJ, Herrstedt J, et al; for the ENGOT-OV16/NOVA investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284. Erratum in: Lancet Oncol. 2017;18:e510.
- Coleman RL, Oza AM, Lorusso D, et al; for the ARIEL3 investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961. Erratum in: Lancet. 2017;390:1948.
- da Cunha Colombo Bonadio RR, Fogace RN, Miranda VC, del Pilar Estevez Diz M. Homologous recombination deficiency in ovarian cancer: a review of its epidemiology and management. Clinics (Sao Paulo). 2018;73(suppl 1):e450s.
- Matsumoto K, Nishimura M, Onoe T, et al. PARP inhibitors for BRCA wild type ovarian cancer; gene alterations, homologous recombination deficiency and combination therapy. Jpn J Clin Oncol. 2019;49:703-707.
- Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27:1449-1455.
- Walker B, Boyd M, Aguilar K, et al. Comparisons of real-world time-to-event end points in oncology research. JCO Clin Cancer Inform. 2021;5:45-46.
- Campbell BA, Scarisbrick JJ, Kim YH, et al. Time to next treatment as a meaningful endpoint for trials of primary cutaneous lymphoma. Cancers (Basel). 2020;12:2311.
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526.
- Karrison T. Restricted mean life with adjustment for covariates. J Am Stat Assoc. 1987;82:1169-1176.