An earlier version of some of the data in this article was included in HOPA News (2021, Volume 18, Issue 1) and was presented at the Annual Hematology/Oncology Pharmacy Association (HOPA) conference on April 13-17, 2021, as a Virtual Conference poster presentation. The abstract was also published in the March 2021 issue of JHOP.
Oral chemotherapy agents continue to be at the forefront of cancer treatment advances and represent up to 35% of all new oncology therapies in the pipeline.1,2 As this trend continues to grow in the coming years, there is an urgent need for institutions to develop standardized processes that maximize the efficacy of these agents while minimizing their adverse events.
Oral therapies are perceived to offer a better quality of life (QOL) compared with intravenous therapies because patients have more flexibility, more autonomy, and less disruption to their daily lives.1,3 However, patients may not realize that choosing an oral therapy regimen shifts many of the responsibilities of adherence and monitoring for adverse events from the oncology team to the patient.3 Ultimately, the benefits of choosing an oral therapy can be greatly overshadowed by decreased efficacy as a result of suboptimal adherence and inadequately managed adverse events.2,4,5 In a randomized study of adherence and symptom management in adults prescribed oral cancer therapies, the mean electronic pill cap adherence rate showed that 85.57% of patients adhered to their therapy, and patients with greater cancer-related symptom severity had lower adherence rates and worsened QOL.5 Similarly, literature on adherence with oral cancer therapies reports that rates of primary nonadherence can be higher than 20%, and an estimated 80% of patients do not adhere to their oral agents according to their physician’s instructions.4,6-8
The stakes for nonadherence for patients with cancer are extremely high and can affect their overall survival and progression-free survival. In a study that evaluated the difference in the 6-year likelihood of major molecular response (MMR) with imatinib treatment in patients with chronic myeloid leukemia, those with a ≥90% adherence rate had an MMR of 94.5% compared with patients with a <90% adherence rate who had an MMR of 28.4%.9,10 The common barriers to adherence include complex administration instructions, limited knowledge about the therapy and adverse events, low health literacy, and financial costs.11 As oral cancer therapies become widely prescribed, institutions must implement standardized processes that systematically address these barriers.
In contrast to the many established safety and quality standards for intravenous cancer treatments, there are currently no standardized guidelines for the outpatient management of patients receiving oral chemotherapy. The available best practice recommendations listed in the Hematology/Oncology Pharmacy Association (HOPA) Pharmacy Standard for Oral Oncolytic Management describe the prescribing of oral cancer agents, patient education, medication dispensing, monitoring, and general management of the clinic.1 The American Society of Clinical Oncology/Oncology Nursing Society Safety Standards recommend safe processes for the ordering, storage, and medication administration of oral cancer drugs.12 Ultimately, institutions must apply these continually evolving best practices to develop their own policies and processes for patient and clinic education and patient monitoring, resulting in immensely varying workflows between institutions.13,14
As part of an interdisciplinary team with nurses, oncologists, and primary care physicians, oncology pharmacists are experts on cancer drugs and can play pivotal roles in outpatient management and leadership of these patients.4,9,15 In a multiple-institution case-control study, patients in the intervention group, who received initial education and ongoing counseling from pharmacists, exhibited an increase in mean daily adherence from 87.2% to 96.8% (P=.03).16 During a 6-month single-institution case-control study, a pharmacist-monitored oral chemotherapy care program resulted in a 59% increase in the detection of drug-related errors (P=.008) and a 20% increase in reported adherence (80.8% vs 60.5%; P<.001).17
In a small-sample, historical-cohort analysis at an outpatient cancer clinic, patients with metastatic, castrate-resistant prostate cancer had significant increases in the number of interventions per patient (6.9 vs 2.6; P=.004) and adherence to laboratory parameter monitoring (10 patients vs 3 patients; P=.04) in the post–program implementation group versus the pre–program implementation group.18
The study by Muluneh and colleagues described an innovative, pharmacy-led, integrated, closed-loop, oral chemotherapy management program for patients with chronic myeloid leukemia, in which pharmacists were able to improve adherence rates and achieve markedly superior early molecular response (88.9% vs 54.8%, respectively; P=.0138) and MMR outcomes (83.3% vs 57.6%, respectively; P=.0575) compared with published literature.19 Although limited by a lack of formal statistical testing and small sample sizes, studies have reported improvements in adherence, and other studies have demonstrated similar improvements through collaborative pharmacist- and nurse-led interventions, such as initial education with ongoing adverse-event and adherence counseling, prefilled pillboxes, and individualized feedback on personal-adherence data.4,20,21
Although available studies suggest positive findings with pharmacist-led initial education and monitoring, a systematic review of published oral chemotherapy intervention programs demonstrates the significant limitations in study design and outcome evaluation in the available literature, ultimately limiting the generalizability of these retrospective and single-center study results.20 Moreover, oncology pharmacists face various financial hurdles of billing at different institutions because of the complexity of assigning a dollar value to the interventions and services they provide.14 These hurdles are exacerbated by a lack of provider status, institution buy-in, and staff availability.14 Mathur and colleagues have demonstrated the value of pharmacist-managed oral chemotherapy at a large institution.22 Yet, prospective studies are needed to further evaluate the outcomes of pharmacist-led oral chemotherapy clinics and potential billing implications.
As a novel way to overcome these challenges and limited resources, this study established a telehealth oral chemotherapy clinic run by a clinical faculty pharmacist contracted with a community cancer center and by pharmacy trainees. If adopted by multiple oncology pharmacy faculty nationwide, a pilot collaborative program of this nature can expand the existing body of literature and provide additional evidence and metrics to support the continued development of pharmacist-led oral chemotherapy clinics.
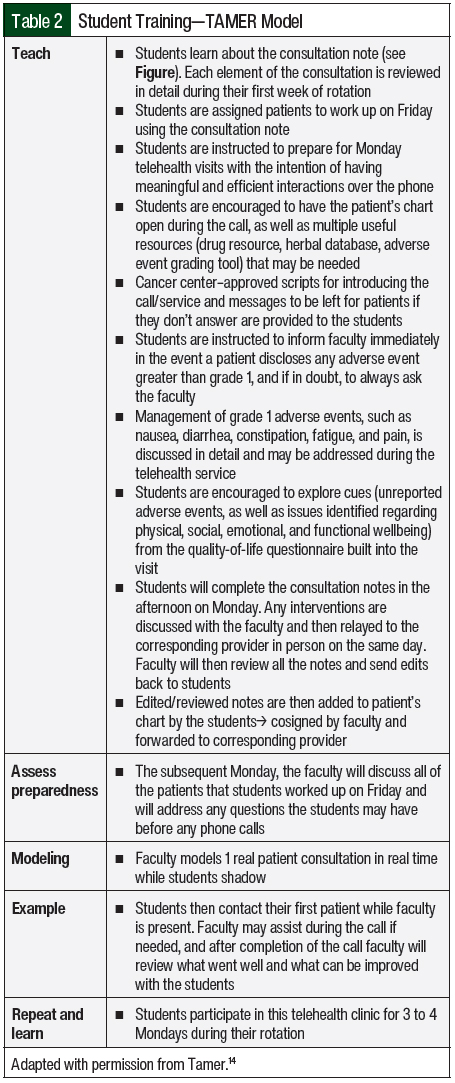
Given the continuous lack of provider status, one of the biggest challenges faced by institutions when implementing new clinical pharmacy services is proving the clinical worth of such services and making the case for a full-time employee despite nonbillable staff time. The primary objective of this study is to describe the development of an oral chemotherapy telehealth clinic and its interventions, which were launched by a clinical oncology pharmacy faculty and pharmacy student trainees at a community cancer center. A secondary objective of this study is to provide a pharmacy student training model—the TAMER model (Teach students, Assess preparedness, Model and encounter, Example completed by students, Repeat and learn; described below and in Table 2)—that can serve as guidance to preceptors at community cancer centers that want to launch a similar initiative with limited resources.
Methods
This retrospective, single-center chart review described and assessed a newly launched oral chemotherapy telehealth clinic. The study was reviewed and determined to qualify for institutional review board (IRB) exemption by the University of Missouri-Kansas City (UMKC) IRB.
The data that were collected included patient demographics (age, sex, race, cancer type, and cancer stage), oral oncolytic type (targeted vs chemotherapy), months since oral therapy initiation, comorbid conditions, number of concomitant medications, QOL Functional Assessment of Cancer Therapy-General (FACT-G; range, 40-108), number of drug interactions identified (drug-drug or drug-food interactions), type of drug interaction identified (increase drug, decrease drug, other), number of interventions per telehealth visit, patient-reported adherence, identified issues with adherence (yes, no, adherence percentage), whether patient was counseled on adherence (yes, no), patient-reported adverse events (oral oncolytic–related, disease state–related, or unrelated), grade of adverse events reported per the Common Terminology Criteria for Adverse Events23 (CTCAE; grade 1, 2, 3, 4, or 5), and type of intervention (referrals to a physical therapist, a primary care doctor for management of comorbidities, a pain specialist, or a social worker or counseling service; initiation of prescription medication; initiating nonpharmaceutical recommendations; switching medications; discontinuing medications; managing treatment-related adverse event; adherence; symptom or disease management; medication reconciliation error; drug interaction; and scheduling laboratory testing per visit).
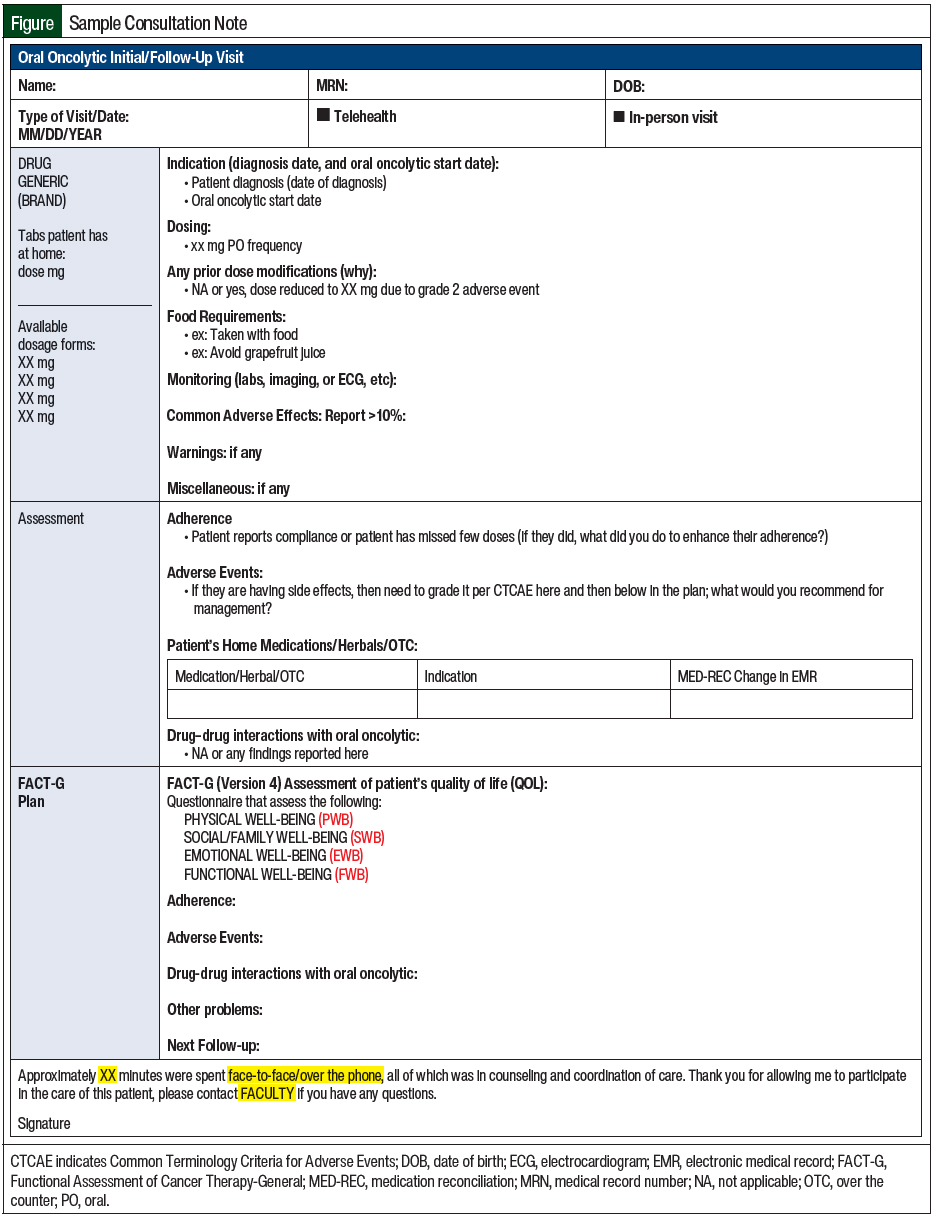
Patient inclusion criteria consisted of adults with cancer (aged ≥18 years) enrolled in the oral chemotherapy telehealth clinic at the cancer center between July 1, 2020, and October 30, 2020. The patient must have received treatment from an oncologist, with complete clinical notes from the provided oncology pharmacy telehealth consultation (Figure).
The oral chemotherapy telehealth clinic was launched by a clinical oncology pharmacy faculty and pharmacy student trainees from the UMKC School of Pharmacy at AdventHealth Cancer Center in Shawnee Mission, KS. Telehealth visits consisted of phone calls with video capability. Telehealth visits were completed by the oncology pharmacy faculty and pharmacy students on oncology rotation.
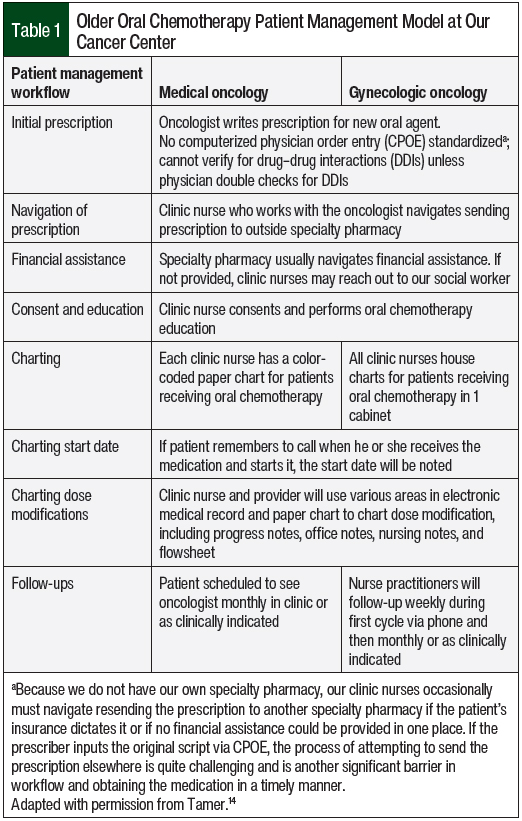
The older chemotherapy patient management model is described in Table 1. The new oral chemotherapy telehealth clinic required switching all patients from paper charts to an online tracker and following up on existing patients via a telehealth visit using the pharmacy consultation vetted by the oncologists at our cancer center (Figure).
Oncologists approved the management of grades 1 and 2 nausea, constipation or diarrhea, skin rash, insomnia, fatigue, and pain directly by pharmacy during telehealth visits. The patients were notified that any other identified adverse events or any grade ≥3 adverse event would be communicated immediately with the preceptor and oncologists. The pharmacists would subsequently communicate with and provide recommendations to oncologists and follow up with the patient to relay their oncologist’s recommendations and follow-up plan. The oncologists also approved any referrals that may have been made by the pharmacists during the telehealth visits as clinically indicated. The pharmacists recommended laboratory testing be ordered or follow-up visits be scheduled with oncologists as clinically necessary. No formal collaborative practice agreement was instituted; this project was presented to the gynecologic oncology and medical oncology groups, where the oral chemotherapy telehealth consultation template (Figure) was approved. Also approved were the parameters for what the pharmacists could do without the oncologists’ approval during those visits and when to reach out to the oncologist. All visits were documented in the electronic medical record (EMR) and were cosigned by the faculty preceptor and the patient’s oncologist.
Fourth-year pharmacy students who were on their advanced pharmacy practice experience month-long oncology rotation took part in the oral chemotherapy clinic. Pharmacy students were assigned patients for workup on Fridays. Morning rounds with the pharmacy faculty were completed on Mondays, followed by telehealth visits. During the visits, if any grade 3 adverse event or any adverse event that was not approved by oncologists to be managed by pharmacy was identified, the pharmacy students would immediately notify the pharmacy faculty; the students were encouraged to have recommendations and discuss them with the faculty. Subsequently, the pharmacy students and faculty discussed interventions with the oncologist and relayed the approved plan and appropriate follow-up to the patient. After the visits were completed, all consultation notes were sent to the pharmacy faculty for edits and were then submitted to the EMR by the students. The notes were cosigned by the pharmacy faculty and the corresponding oncologist.
The pharmacy students were trained using the TAMER model, which consists of teaching students, assessing preparedness, modeling an encounter, having an example completed by the students, and repeating and learning. The first step is teaching the students about the importance of the clinic, the history behind it, and why it was established. The students are taught about each part of the pharmacy consultation (Figure) and what information to collect on Fridays before conducting the telehealth visits on Mondays. They are also taught how to grade adverse events and how to manage oncologist-approved grades 1 and 2 nausea, constipation or diarrhea, skin rash, insomnia, fatigue, and pain during the telehealth visits. The students are assessed on their preparedness and any questions they have are answered during Monday rounds before the telehealth visits. An example telehealth visit is modeled to the students before their first telehealth encounter. Examples are completed by the students while the faculty is present during the entire encounter. These steps are repeated and the students continue to learn and improve through 3 to 4 scheduled Monday clinics during the month-long oncology rotation. The TAMER model is described in more detail in Table 2.
Results
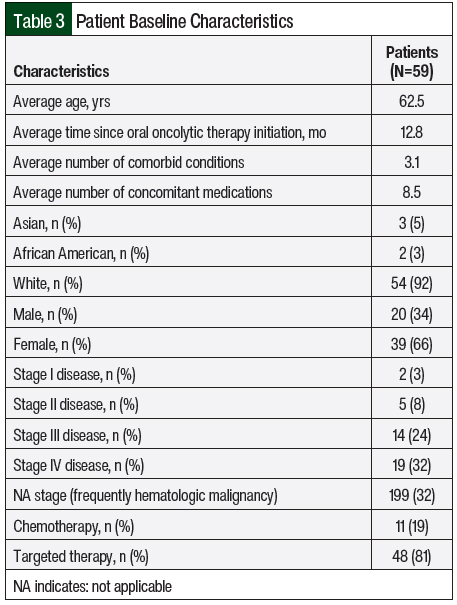
Over the course of 4 months, 59 charts belonging to patients who underwent a telehealth visit were reviewed. Patients were predominantly elderly and had multiple comorbidities, with an average age of 62.5 years and 3.1 comorbid conditions per patient. Stage IV disease was the most common (32%), and a vast majority of patients were receiving oral targeted therapy (81%) compared with oral chemotherapy (19%). All other baseline characteristics are listed in Table 3.
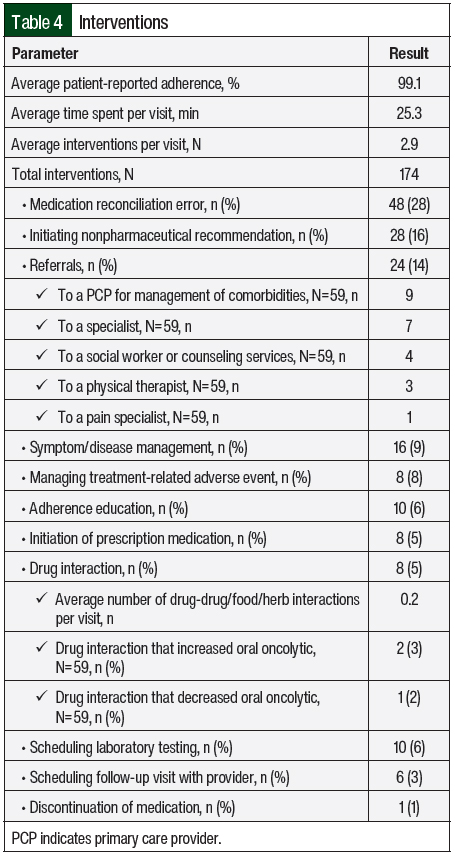
An average of 2.9 interventions per visit was noted, with an average time of 25.3 minutes spent with the patients per visit. Of the 174 total interventions, the most common were medication reconciliation error (28%), the initiation of nonpharmacologic therapy (16%), and referrals (14%). All interventions are broken down in Table 4. Referral to the patient’s primary care provider for the management of a comorbidity (15%) and referral to a specialist (12%) were the most common referrals. A total of 58 adverse events were identified, and 34 adverse events were managed and graded per the CTCAE23 (grade 1, 22; grade 2, 11; grade 3, 1). Fatigue, insomnia, and depression were captured using the FACT-G QOL questionnaire and were not graded. Fatigue and insomnia were managed during the telehealth visit, and depression was designated for referral. The 58 total adverse events included fatigue (n=17, 29%), other (n=15, 26%), nausea (n=7, 12%), insomnia (n=6, 10%), peripheral neuropathy (n=3, 5%), diarrhea (n=3, 5%), rash (n=3, 5%), hepatotoxicity (n=2, 3%), depression (n=1, 2%), and hypertension (n=1, 2%).
Approximately 0.2 drug interactions were identified per visit, with 2 interactions increasing the oral oncolytic drug and 1 interaction decreasing exposure to the oral oncolytic drug. Patient-reported adherence was extremely high, with an average of 99.1% adherence to the oral oncolytic drug, but 20% of patients were still identified and counseled on adherence.
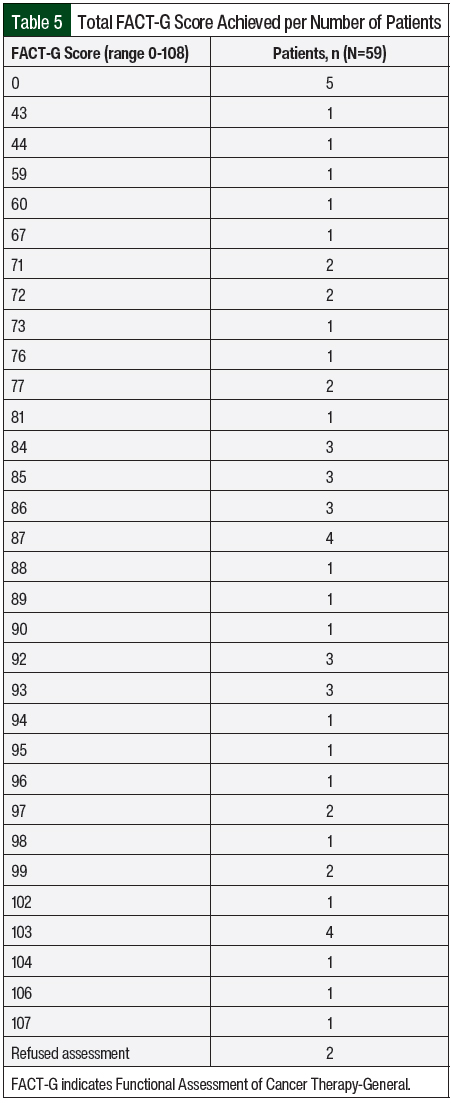
The FACT-G scores were collected and are shown in Table 5. FACT-G is a 27-item questionnaire that was part of the oral chemotherapy telehealth clinic visit to assess the QOL of patients with cancer while receiving therapy.24 The FACT-G is composed of 4 subscales, including physical well-being (score range, 0-28), social/family well-being (score range, 0-28), emotional well-being (score range, 0-24), and functional well-being (score range, 0-28). All questions use a 5-point rating scale (0 = not at all; 1 = a little bit; 2 = somewhat; 3 = quite a bit; and 4 = very much). The total score is calculated as the sum of the 4 scores (score range, 0-108 points). Patients could choose to opt out of the survey, and if they did so, their score was left blank. Higher scores indicate better QOL. The FACT-G score allowed the capture of adverse events not otherwise reported by patients that could impact their QOL, such as lack of sleep. Those adverse events were managed during the visit or the patient was referred to a specialist.
Discussion
The implementation of a pharmacist-led, standardized oral chemotherapy telehealth clinic resulted in multiple interventions per patient visit. These interventions improved patients’ adherence to best practice recommendations set forth by HOPA, which describe standards for follow-up, laboratory monitoring, and adverse-event management. Pharmacists and students performed timely medication reconciliations and assessed drug-drug and drug-food interactions during each patient visit. Moreover, they were able to fill gaps in medication education and serve as facilitators between the patient and provider to address medication concerns or errors. The management of grades 1 and 2 adverse events during the telehealth visit may have prevented further progression to a higher-grade adverse event. Higher-grade adverse events were managed by the pharmacy team after discussion with an oncologist, and follow-up with the patient was conducted by the pharmacy team, decreasing the oncologists’ burden or the need to conduct the follow-up themselves. The oncologists had access to these consultations before subsequent patient visits, which may have helped shorten their previsit workup. Even for patients who reported high adherence rates, the pharmacist was frequently able to intervene in detecting and managing adverse events and other therapy-related difficulties that might have otherwise gone unnoticed. All these interventions are vital to improved patient outcomes and patient QOL and are supported by best practices in the management of oral oncolytic therapy.1
The important role of clinical oncology pharmacists has been discussed in numerous articles, specifically in the realm of oral oncolytic management.1,25,26 With the rapidly growing landscape of oral treatment options for patients with cancer, pharmacists serve a critical role in assisting patients and providers in selecting the optimal agent, educating all parties involved on that agent, and monitoring for adverse events. These qualities make pharmacists a valuable addition to a patient’s care team and qualify pharmacists to lead outpatient oral oncolytic clinics in person or via telehealth. The implementation of and justification for such clinics continue to be a struggle because of the lack of provider status and ability to bill for services, and the lack of large prospective studies to evaluate the cost-savings associated with pharmacist-led services and interventions.
The HOPA Pharmacy Practice Standard for the Management of Oral Oncolytic Therapy recommends frequent and detailed follow-up and assessment of patient adherence, symptoms, and education at least once at the initiation of therapy as well as before each medication refill.1 These activities require time and detailed patient workup, which currently are not pharmacist-billable activities and, as a result, are financially unjustified in many settings, such as smaller institutions with limited clinical pharmacists. Championed by national pharmacy organizations such as the American Pharmacists Association, provider status remains a top priority for pharmacists. The passing and enactment of HR 2759/S 1362, the Pharmacy and Medically Underserved Areas Enhancement Act, which was introduced in April 2021, would recognize pharmacists as providers and would allow for the billing of services provided to patients in medically underserved communities under Medicare Part B.27 This bill would be the first step in allowing clinical pharmacists trained in oral chemotherapy management to justify providing these services and thus improve the care of and outcomes for patients.
Limitations
There are several limitations to this study that are consistent with the challenges reported in existing literature and by institutions implementing similar clinics.
First, the fairly small sample size is only representative of our center’s patients and may not be generalizable to the prospective patients for this type of service nationwide. Although this study will add to the numerous reported positive pharmacist interventions in the current literature, larger prospective, multicenter studies are needed to quantify the true monetary value and impact of pharmacist-led oral oncolytic drug clinics for patients across different cancer populations.
Second, the self-reported adherence rates in this study are much higher than those in the current literature (99% vs approximately 80%).4,6-8 This information is highly subjective and is difficult to verify because most EMRs do not provide comprehensive prescription fill histories or prescription insurance claims data. By maintaining consistent follow-up and building patient-pharmacist rapport through a telehealth service, patients may be more willing to discuss barriers to adherence and plans for improvement.
Conclusions
This study demonstrates that an oral chemotherapy clinic can be developed and managed by an oncology pharmacist with very limited resources using the TAMER model. Establishing a standardized clinic to manage and follow up with patients with cancer who receive oral oncolytics may improve patients’ outcomes and serve as a training and learning avenue for pharmacy student trainees. This clinic has been ongoing since 2020 and has been appreciated by the providers at our cancer center, pharmacy students on oncology rotation, as well as patients receiving this free service.
After the COVID-19 pandemic, during which cancer screenings were significantly reduced, more patients may receive an advanced cancer diagnosis and may qualify for oral oncolytic treatments, which could expand the number of patients receiving these drugs. Oncology pharmacists are pivotal healthcare members and treatment experts on the cancer care team that may help manage these patients. This study demonstrated the value and interventions that such an initiative can provide to patients.
Author Disclosure Statement
Dr Tamer, Dr Schieber, Dr Wodtke, and Dr Zantout have no conflicts of interest to report.
References
- Mackler E, Segal EM, Muluneh B, et al. 2018 Hematology/Oncology Pharmacist Association best practices for the management of oral oncolytic therapy: pharmacy practice standard. J Oncol Pract. 2019;15:e346-e355.
- DeCardenas R, Helfrich JS. Oral therapies and safety issues for oncology practices: patient support is key to delivering safe oral chemotherapy. Oncol Issues. 2010;25:40-42.
- Weingart SN, Brown E, Bach PB, et al. NCCN Task Force Report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(suppl 3):S-1–S-14.
- D’Amato S. Improving patient adherence with oral chemotherapy. Oncol Issues. 2008;23:42-45.
- Jacobs JM, Ream ME, Pensak N, et al. Patient experiences with oral chemotherapy: adherence, symptoms, and quality of life. J Natl Compr Canc Netw. 2019;17:221-228.
- Mancini R, McBride A, Kruczynski M. Oral oncolytics: part 1-financial, adherence, and management challenges. Oncology (Williston Park). 2013;27:742-744, 824.
- Gellad WF, Grenard J, McGlynn EA. A review of barriers to medication adherence: a framework for driving policy options. RAND Corporation; 2009. www.rand.org/pubs/technical_reports/TR765.html. Accessed August 17, 2022.
- Jabbour EJ, Kantarjian H, Eliasson L, et al. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol. 2012;87:687-691.
- Thomas SA, John T, Criner E, Nguyen TM. Challenges to oral chemotherapy adherence. US Pharm. 2019;44:HS-9–HS-12.
- Marin D, Bazeos A, Mahon F-X, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381-2388.
- Jin J, Sklar GE, Oh VMS, Li SC. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4:269-286.
- Neuss MN, Gilmore TR, Belderson KM, et al. 2016 Updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards, including standards for pediatric oncology. J Oncol Pract. 2016;12:1262-1271. Erratum in: J Oncol Pract. 2017;13:144.
- Fahrenbruch B, Griffith N, Hari P, et al. Steps to Success: Implementing Oral Oncolytics. Association of Community Cancer Centers; September 2016. www.accc-cancer.org/docs/projects/pdf/implementing-oral-oncolytics-final.pdf. Accessed August 17, 2022.
- Tamer D. Launching an oral chemotherapy telehealth clinic using the TAMER Model. HOPA News. 2021;18:3-5.
- Gaertner K. The role of the pharmacist in managing oral chemotherapy. Pharm Purch Prod. 2019;16:18-22.
- Simons S, Ringsdorf S, Braun M, et al. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer. 2011;19:1009-1018.
- Ribed A, Romero-Jiménez RM, Escudero-Vilaplana V, et al. Pharmaceutical care program for onco-hematologic outpatients: safety, efficiency and patient satisfaction. Int J Clin Pharm. 2016;38:280-288.
- Patel JM, Holle LM, Clement JM, et al. Impact of a pharmacist-led oral chemotherapy-monitoring program in patients with metastatic castrate-resistant prostate cancer. J Oncol Pharm Pract. 2016;22:777-783.
- Muluneh B, Schneider M, Faso A, et al. Improved adherence rates and clinical outcomes of an integrated, closed-loop, pharmacist-led oral chemotherapy management program. J Oncol Pract. 2018;14:e324-e334.
- Zerillo JA, Goldenberg BA, Kotecha RR, et al. Interventions to improve oral chemotherapy safety and quality: a systematic review. JAMA Oncol. 2018;4:105-117.
- Irwin M, Johnson LA. Factors influencing oral adherence: qualitative metasummary and triangulation with quantitative evidence. Clin J Oncol Nurs. 2015;19(3 suppl):6-30.
- Mathur AD, Maiers TA, Andrick BJ. Impact of a pharmacist-led telehealth oral chemotherapy clinic. Am J Health Syst Pharm. 2022;79:896-903.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. November 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed August 17, 2022.
- Fairclough DL, Cella DF. Functional Assessment of Cancer Therapy (FACT-G): non-response to individual questions. Qual Life Res. 1996;5:321-329.
- Bowles H, Tawfik B, Abernathy J, et al. Pharmacist-driven oral oncolytic medication education and consent. JCO Oncol Pract. 2020;16:e1209-e1215.
- Wright AL, Matta SF, Kerr JR. Expansion of pharmacist practice in oral oncolytic therapy with a collaborative practice agreement. J Oncol Pharm Pract. 2020;26:1886-1893.
- American Pharmacists Association. Pharmacy’s Top Priority: Medicare Provider Status Recognition. https://pharmacist.com/Advocacy/Issues/Medicare-Provider-Status-Recognition. Accessed August 17, 2022.