Although tyrosine kinase inhibitors (TKIs) are tolerated relatively well in patients with chronic myeloid leukemia (CML),1-7 there is a wide range of adverse events that make indefinite therapy with TKIs challenging for some patients.8,9 The risks for long-term TKI-related adverse events, including pulmonary hypertension and pleural effusion with dasatinib treatment and vascular events with nilotinib and ponatinib treatments, are not trivial.10,11 Furthermore, patients with cancer often have burdensome costs associated with TKI-related co-payments, coinsurance, and deductibles.12 Thus, the discontinuation of TKI therapy for some patients with CML could relieve financial toxicity and could prevent the onset of short- and long-term adverse events.
Guidelines are available that provide recommendations for the management of patients with CML, optimal response to therapy, and considerations for TKI discontinuation. The National Comprehensive Cancer Network (NCCN)13 and the European Society for Medical Oncology14 are 2 organizations that have guidelines that recommend when to consider the discontinuation of TKI therapy. The NCCN’s Clinical Practice Guidelines in Oncology (NCCN Guidelines) are updated several times annually, and its recommendations for monitoring patients after TKI discontinuation have changed in recent years.13 Version 3.2022 of the NCCN’s guidelines for patients with CML includes TKI discontinuation criteria for patients who meet the following requirements: aged ≥18 years, have chronic-phase CML (no history of accelerated-phase or blast-phase CML), have received TKI therapy for at least 3 years, have quantifiable BCR-ABL1 transcript, and have had a stable molecular response (MR4; BCR-ABL1 ≤0.01% international scale [IS]) for ≥2 years on at least 4 tests performed at least 3 months apart.13 After TKI discontinuation, BCR-ABL1 monitoring is recommended once or twice monthly for the first 6 months, bimonthly in months 7 to 12, and every 3 months thereafter indefinitely.13 The resumption of treatment is recommended within 4 weeks for any patients who are unable to maintain a major molecular response, which is defined as MR3 (BCR-ABL1 ≤0.1% IS). Because there are risks for disease relapse and other adverse events, such as withdrawal syndrome, a formalized consent conversation is recommended for all patients with CML who are considering TKI discontinuation.13
TKI discontinuation in patients with CML has been studied in several clinical trials, where approximately 50% (range, 38%-61%) of patients who discontinued treatment with imatinib, dasatinib, or nilotinib were able to remain in treatment-free remission ranging from 6 to 60 months.15-17 Despite these convincing clinical trial data for the discontinuation of imatinib, dasatinib, and nilotinib, the outcomes are lacking for bosutinib, ponatinib, and asciminib treatment discontinuation. In addition, there is limited outcomes information on TKI discontinuation among patients with CML in real-world settings, especially when the initiation of the TKI discontinuation is by a nonphysician.18-20
With the emergence of guidelines that support the discontinuation of TKI therapy in patients with CML, oncology clinical pharmacy specialists (CPSs) in collaboration with oncologists at 2 Kaiser Permanente regions, Kaiser Permanente Colorado (KPCO) and Kaiser Permanente of the Northwest (KPNW), developed TKI discontinuation programs for patients with CML who meet the NCCN’s guideline criteria.13 CPSs have demonstrated abilities in assisting physicians in the optimization of medication management, including medication discontinuation.21,22 The purpose of this analysis of real-world patients was to examine the clinical and financial impacts of a CPS-initiated TKI discontinuation process in patients with CML. This assessment can provide oncology practitioners, policymakers, patients, and caregivers with information on the feasibility, successes, and shortcomings of TKI discontinuation in a real-world, non–clinical trial setting.
Methods
This retrospective, descriptive analysis included patients receiving a TKI for the treatment of CML between January 1, 2019, and September 30, 2020. The included patients were categorized as candidates and noncandidates for TKI discontinuation. The index date was the date a patient was assessed for TKI discontinuation candidacy. The patients were followed until December 31, 2020, or until their health plan membership termination to assess for TKI discontinuation and patient outcomes.
The nonprofit, integrated healthcare delivery systems KPCO and KPNW serve patients in the Front Range of Colorado and Oregon and southwestern Washington states, respectively, with a combined membership of approximately 1.2 million persons. The members are served at the systems’ 91 clinics that provide primary and specialty (including oncology) care and pharmacy services and at KPNW’s 2 hospitals. Kaiser Permanente uses an electronic health record (EHR; EPIC Systems Corp) where data are housed on office, telephonic, virtual, and email visits regarding laboratory, medication, procedure, imaging, and other encounter-related information. In addition, coded and free-text emergency department, hospitalization, and membership information from within the delivery system, as well as from other contracted and affiliated facilities, are captured in Kaiser Permanente’s electronic administrative and claims databases. All aspects of this analysis were reviewed and approved by each health plan’s institutional review board before the data collection.
Patient Population
The study patients were identified from the systems’ CPS TKI tracking spreadsheet. The included patients were aged ≥18 years as of the index date, were diagnosed with CML, were receiving TKI therapy (with bosutinib, dasatinib, imatinib, nilotinib, or ponatinib) for CML, and had 6 months of continuous membership before the index date (to assure the adequate capture of their clinical profiles). A patient was identified as a TKI discontinuation candidate if he or she had chronic-phase CML with no history of accelerated- or blast-phase CML, had CML and no previous TKI discontinuation attempted for stable disease, received TKI therapy for ≥3 years, and had a stable molecular response (BCR-ABL1 ≤0.01% IS for ≥2 years as documented by ≥4 tests recorded ≥3 months apart). A patient was identified as a future TKI discontinuation candidate if they were on track to meet all of the candidate eligibility requirements during the next 3 years after screening.
Intervention
In KPCO, CPSs led the development of a program to systematically identify all patients who received a TKI for any indications. The program included educational presentations to oncologists on the clinical guidelines for TKI discontinuation in patients with CML. CPSs and oncologists collaborated to create note templates and TKI discontinuation consent forms to standardize the documentation and patient counseling. Starting in 2019, CPSs performed quarterly reviews to identify the patients with CML who were receiving TKI therapy and placed them on a “shared reminder list” within the EHR. The list’s functionality includes provider reminder alerts, temporal milestones to assess the BCR-ABL1 results, and the capabilities for the assessment of TKI discontinuation candidacy per the NCCN guidelines.13
At KPNW, a similar approach to patient identification was conducted, except an analyst created a list of patients with CML and an active TKI prescription quarterly using administrative data. A CPS conducted a manual review of an identified patient’s chart to determine his or her TKI discontinuation candidacy per the NCCN guidelines.13
After identifying a patient who met the TKI discontinuation criteria, the CPS reached out to the patient’s oncologist to discuss the discontinuation of TKI treatment. The oncologist reviewed each patient’s clinical profile, and if they concurred that the patient was appropriate to discontinue the TKI, they consulted with and consented the willing patients. After the discontinuation of TKI treatment, the CPS consulted with oncologists on laboratory test monitoring adherence and result interpretations, thresholds for restarting TKI therapy, and TKI withdrawal symptoms management. All communications and decisions were recorded as clinical notes in the EHR.
The primary study outcomes were the counts of patients who were existing TKI discontinuation candidates and who discontinued TKI therapy. The secondary outcomes included the counts of patients who were screened for TKI discontinuation, were non–TKI discontinuation candidates, were future TKI discontinuation candidates, were non–future TKI discontinuation candidates, had their managing oncologist agree with the pharmacist’s recommendation that they were a TKI discontinuation candidate, and had consented to TKI discontinuation. Among the patients who discontinued TKI treatment, the counts of patients who discontinued receiving a TKI without meeting the screening criteria; who remained off of therapy and the duration they were off of therapy; who resumed therapy, including what therapy was resumed and the duration they were off of therapy before resuming therapy; who were adherent to BCR-ABL monitoring; who had TKI discontinuation syndrome (ie, musculoskeletal pain); and, for patients who resumed therapy, who achieved the resumption of MR4 response and time to achieving a response were compiled and the percentages were noted. Data on the criteria that were not met for candidacy, the reasons for TKI resumption, and the TKI costs avoided are reported here.
Adherence to BCR-ABL monitoring was defined as the monthly monitoring of BCR-ABL for the first 6 months, bimonthly in months 7 to 12, and every 3 months thereafter.13 Cost avoidance was based on the TKI’s wholesale acquisition cost (WAC)23 as of January 19, 2023.
Information for all of the patients retrieved from the shared reminder list (KPCO) and analyst reports (KPNW) was collected via manual chart reviews by 4 authors (JMF, YMB, LAT, and EE). The information that was collected included the indication and diagnosis date; current and previous CML phase(s); birth date; death date (as applicable); sex; race; ethnicity; TKI, dose, and dates of TKI use; BCR-ABL1 values and dates; the managing oncologist’s agreement status; the patient’s consent status; the criteria that were not met for candidacy; the reasons for resumption; and the TKI discontinuation syndrome status.
Age was calculated as of the date of the index TKI initiation. TKI discontinuation candidacy was assessed between January 1, 2019, and September 30, 2020, with follow-up for TKI resumption and BCR-ABL1 monitoring through December 31, 2020. The duration (in years) since diagnosis and the index TKI initiation was calculated as of September 30, 2020, minus the respective date. The duration (in weeks) off of therapy was calculated as the date of the TKI resumption or December 31, 2020, whichever came first, minus the date of the TKI discontinuation. The costs avoided were calculated with the milligram dose form– and strength–specific WAC of each patient’s daily dose for his or her most recent TKI dispensed before the TKI discontinuation. The cost of the daily dose was then multiplied by the total number of days off of therapy or until the end of the study period, whichever came first.
The patients’ characteristics and outcomes are reported as means with standard deviations and medians with interquartile ranges (IQRs), as appropriate, for interval- and ratio-level variables (eg, age, costs avoided) and counts and proportions for categorical variables (eg, sex).
Results
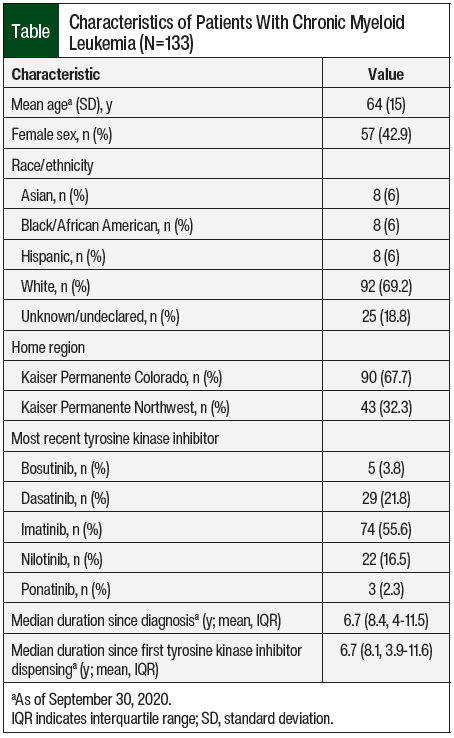
A total of 255 patients who were receiving an eligible TKI were screened, and 133 (52.2%) had a diagnosis of CML. The study patients were primarily older adults, male, and white, and had initiated TKI therapy >6 years before the index date (Table). Overall, 36 patients discontinued TKI therapy, including 23 who met guidelines and 13 who discontinued on their own, and 15 of the 36 (41.7%) resumed therapy. All of the patients who resumed therapy did so as a result of loss of response. A total of 41 (30.8%) patients were identified as TKI discontinuation candidates by the CPS, and 23 (56.1%) of these patients discontinued therapy after their managing oncologist agreed and the patient consented to the discontinuation of the TKI (Figure 1).
Among the 23 patients identified as TKI discontinuation candidates by the CPS who consented to and discontinued TKI therapy after their managing oncologist agreed to the discontinuation, 18 (78.3%) remained off of TKI therapy through December 31, 2020. The median duration off of therapy was 59 weeks (IQR, 51-86), 47.8% were adherent to BCR-ABL1 monitoring, and 13% had TKI discontinuation syndrome. Among the 5 patients identified as TKI discontinuation candidates by the CPS who consented to and discontinued TKI therapy after their managing oncologist agreed to their discontinuation and then resumed TKI therapy, the median duration off of therapy was 15 weeks (IQR, 11-26). All 5 of these patients achieved MR4 and resumed the TKI that they had initially discontinued.
The majority of patients (n=87; 94.6%) who were not TKI discontinuation candidates (n=92) were identified as future TKI discontinuation candidates (Figure 1). The patients who did not meet the criteria for TKI discontinuation included 6 (6.5%) with a history of accelerated- or blast-phase CML, 3 (3.3%) with no quantifiable BCR-ABL1 transcript, 55 (59.8%) with no response of MR4 or better for 2 years, 21 (22.8%) who received TKI treatment for <3 years, and 7 (7.6%) with other criteria.
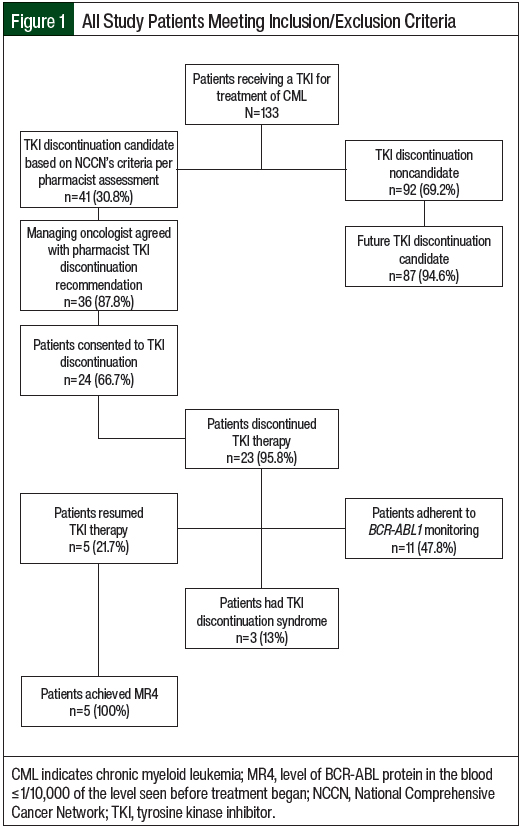
In all, 13 (36.1%) of the 36 patients who discontinued TKI therapy were not TKI discontinuation candidates and discontinued TKI therapy on their own (Figure 2). Among these 13 patients, 3 (23.1%) remained off of therapy through follow-up, 5 (38.4%) adhered to BCR-ABL1 monitoring, and none had TKI discontinuation syndrome; the median duration off of therapy was 20 weeks (IQR, 13-27). Of the 10 patients who resumed TKI therapy, 6 (60%) achieved MR4 and 7 (70%) resumed the TKI that they had initially discontinued; the median duration off of therapy was 15 weeks (IQR, 11-23).
Among the 23 patients who consented to and then discontinued TKI therapy, 1 (4.4%) discontinued bosutinib, 6 (26.1%) discontinued dasatinib, 7 (30.4%) discontinued imatinib, and 9 (39.1%) discontinued nilotinib. For these patients, the median TKI therapy cost avoided per patient was $148,462 (IQR, $2167-$238,579), and the total cost avoided was $3,485,642. Among the 13 patients who were not discontinuation candidates but who discontinued TKI therapy, 5 (38.5%) discontinued dasatinib, 7 (53.9%) imatinib, and 1 (7.7%) discontinued nilotinib. For these patients, the median TKI therapy cost avoided per patient was $791 (IQR, $432-$46,390) and the total cost avoided was $447,044. Among all 36 patients who discontinued TKI therapy, the median TKI therapy cost avoided per patient was $45,587 (IQR, $782-$178,520) and the total cost avoided was $3,932,686.
Discussion
This real-world, descriptive analysis of 133 patients with CML who were screened for TKI discontinuation showed that pharmacist-initiated TKI discontinuation programs were able to identify existing and future TKI discontinuation candidates based on the NCCN’s guidelines.13 Approximately 33% of the patients screened by a CPS were eligible for TKI discontinuation, and TKI therapy was discontinued in more than half of these patients. In addition, pharmacist screening identified that the vast majority of patients could become candidates for TKI discontinuation in the future. Furthermore, our findings indicate that substantial TKI costs can be avoided with minimal adverse events from TKI discontinuation when patients meet the recommended criteria for TKI discontinuation. Nevertheless, patients who do not meet the recommended criteria are unlikely to remain off of therapy, to achieve significant cost-savings, and may have fewer deep responses when TKI therapy is resumed. Overall, our findings are important because they provide evidence that clinical pharmacists, as oncologist extenders, may be suitable to collaborate actively in the management of the TKI discontinuation process in patients with CML. These findings suggest that patients with CML who are receiving a TKI could be screened by a clinical pharmacist to identify opportunities for TKI discontinuation.
We identified that 96% of patients who were offered TKI discontinuation accepted to do so, which is a numerically higher rate than reported in a study by Ferrero and colleagues (85%).19 Whereas we identified that approximately 31% of patients with CML who were receiving a TKI were existing TKI discontinuation candidates and that the vast majority of patients who were not existing TKI discontinuation candidates may be in the future, Horn and colleagues reported that only 14% of patients would be a TKI discontinuation candidate based on their modeling.26 The rate of 58% of patients who discontinued treatment with and remained off of TKI therapy in our analysis is in alignment with rates in other TKI discontinuation trials15-17,24,25 and real-world analyses.18-20 We identified numerically similar durations off of treatment (median, 15 weeks) in the patients who resumed TKI therapy in other real-world analyses (the majority resumed TKI treatment within 6 months of discontinuation).18,20 We identified that 11 of 15 (73%) patients overall who discontinued and then resumed TKI therapy achieved MR4. Similar to our findings, Takahashi and colleagues reported that of the 17 patients who discontinued and then resumed TKI therapy, 77% achieved MR4.18 They did not report the duration until achieving MR4.18 The overall proportion of patients with TKI discontinuation syndrome in our study was numerically lower than the proportion reported in the study by Hernández-Boluda and colleagues (8% vs 22%, respectively); however, this may be related to our study’s shorter follow-up (median, 15 months vs 21 months, respectively).20
To our knowledge, there are no published real-world studies to assess the proportion of patients who adhere to BCR-ABL1 monitoring after the discontinuation of TKI therapy or TKI costs avoided. It is notable that our study showed modest adherence with follow-up BCR-ABL1 monitoring (44%). Anecdotally, we believe this to be negatively impacted by the COVID-19 pandemic, during which patients chose to avoid perceived elective trips to healthcare facilities. We estimated that the TKI costs avoided exceeded $98,000 per patient based on the January 19, 2023, WAC. In their 2018 prospective trial, Saussele and colleagues estimated the TKI costs avoided to be >$46,000 (in 2023 US dollars) per patient who discontinued TKI therapy.16 Taken together, these findings suggest a substantial reduction in healthcare costs with TKI discontinuation.
It was surprising that 13 (approximately 10% of all patients in our analysis) patients self-discontinued treatment with a TKI independent of the pharmacist-initiated program. Unfortunately, we did not collect information on why the TKI self-discontinuation occurred because it was not typically documented. The demographic data in our study indicate that the patients with CML are largely representative of a typical population with CML in the United States,27 suggesting that TKI self-discontinuation could occur in a sizable number of patients with CML. Nevertheless, TKI self-discontinuation remains an understudied phenomenon28 that is not supported by our findings.
The successes of the pharmacist-initiated TKI discontinuation programs are likely attributable to our institutions’ adherence to the NCCN’s guidelines on TKI discontinuation candidacy.13 Close monitoring of patients who are being considered for TKI discontinuation was a key factor in determining if a patient is a TKI discontinuation candidate. Although hematologic and cytogenetic testing are key components in the initial phases of CML treatment, monitoring molecular response is required to monitor the deep phases of response during TKI treatment candidacy screening.13 In addition, the collaborations between the oncologist and the CPS to identify patients and obtain consent before TKI discontinuation along with the CPS’s use of reminders within the EHR and observing follow-up monitoring could have led to long-term TKI therapy discontinuation. This is supported by our observation of a numerically lower proportion of patients with long-term therapy discontinuation who self-discontinued TKI treatment without meeting the established discontinuation criteria. Future enhancements to the program are warranted with personnel (eg, telephone consultations) and technology (eg, text messaging), with patient-centered solutions being considered to improve monitoring and to identify patients who are at risk for the self-discontinuation of TKI treatment.
Limitations
Our analysis has limitations. We did not have a control group to compare our outcomes against. The WACs that we used for the TKIs are lower than what a typical health plan pays for a TKI. We did not account for patient-specific, practitioner, or TKI discontinuation syndrome costs nor for the cost of polymerase chain reaction (PCR) testing after the TKI discontinuation in the costs-avoided analysis. It is reasonable that testing is more frequently performed during the initial period after TKI discontinuation. Because recommended PCR testing results in fewer complications, the transient increase in testing frequency is unlikely to contribute to an increased cost in care.29
Nevertheless, a formal economic analysis with a larger patient population is warranted, particularly including nonintegrated healthcare delivery systems. As a result of the real-world, retrospective nature of our analysis, the follow-up varied by patient. Adherence to postdiscontinuation BCR-ABL1 was lower than we anticipated, and the COVID-19 pandemic may have contributed; this is an important area of focus for future TKI discontinuation programs. Because patient self-discontinuation of treatment with a TKI was not expected to occur with meaningful frequency, we were not able to collect information on reasons why this occurred.
Conclusion
During the past 20 years, treatments for CML have changed dramatically, with vastly improved outcomes, most recently the possibility of TKI discontinuation to minimize the long-term adverse events of treatment without compromising the patients’ outcomes. Our pharmacist-initiated TKI discontinuation programs for patients with CML, using systematic screening and collaboration with oncologists to formally discontinue TKI treatment, promoted safe follow-up while achieving TKI costs avoided.
Because it may be challenging for managing oncologists with a large patient panel to identify their patients’ TKI discontinuation candidacy and status, a pharmacist-initiated program offers to extend the oncologists’ ability to deliver a patient-centered approach that provides benefit to the patient and to the healthcare system. Future studies should be conducted to better characterize the cost-savings, test the efficacy of EHR tools, and use clinical pharmacists to further aid in TKI discontinuation programs and postdiscontinuation BCR-ABL1 monitoring.
Funding Source
This analysis was funded by Kaiser Permanente.
Author Disclosure Statement
Dr Byakina was an employee of Kaiser Permanente during the study; Dr Freml, Dr Thompson, Dr Ekinci, Dr Knudsen, Dr De Silva, Dr Boyle, and Dr Delate have no conflicts of interest to report.
References
- Gleevec (imatinib mesylate) tablets, for oral use [prescribing information]. Novartis; August 2022. www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/gleevec_tabs.pdf. Accessed October 25, 2023.
- Sprycel (dasatinib) tablets, for oral use [prescribing information]. Bristol-Myers Squibb Company; February 2023. https://packageinserts.bms.com/pi/pi_sprycel.pdf. Accessed October 25, 2023.
- Tasigna (nilotinib) capsules, for oral use [prescribing information]. Novartis; September 2021. www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/tasigna.pdf. Accessed October 25, 2023.
- Bosulif (bosutinib) tablets/capsules, for oral use [prescribing information]. Pfizer; September 2023. http://labeling.pfizer.com/ShowLabeling.aspx?id=884. Accessed October 25, 2023.
- Iclusig (ponatinib) tablets, for oral use [prescribing information]. Ariad Pharmaceuticals; February 2022. www.iclusig.com/pdf/ICLUSIG-Prescribing-Information.pdf. Accessed October 25, 2023.
- Synribo (omacetaxine mepesuccinate) for injection, for subcutaneous use [prescribing information]. Cephalon; May 2021. www.synribohcp.com/globalassets/synribo-hcp/downloadable-assets/synribo_pi.pdf. Accessed October 25, 2023.
- Scemblix (asciminib) tablets, for oral use [prescribing information]. Novartis; June 2023. www.novartis.us/sites/www.novartis.us/files/scemblix.pdf. Accessed October 25, 2023.
- Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186-e193.
- Williams LA, Garcia Gonzalez AG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122:641-647.
- Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119:1123-1129.
- Chai-Adisaksopha C, Lam W, Hillis C. Major arterial events in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a meta-analysis. Leuk Lymphoma. 2016;57:1300-1310.
- National Cancer Institute. Financial toxicity and cancer treatment (PDQ)–health professional version. Updated September 20, 2022. www.cancer.gov/about-cancer/managing-care/track-care-costs/financial-toxicity-hp-pdq. Accessed October 25, 2023.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Chronic Myeloid Leukemia. Version 3.2022. Accessed February 24, 2022.
- Hochhaus A, Saussele S, Rosti G, et al; for the ESMO Guidelines Committee. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv41-iv51. Erratum in: Ann Oncol. 2018;29(suppl 4):iv261.
- Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298-305.
- Saussele S, Richter J, Guilhot J, et al; for the EURO-SKI investigators. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747-757.
- Mahon FX, Boquimpani C, Kim DW, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168:461-470.
- Takahashi N, Kyo T, Maeda Y, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97:903-906.
- Ferrero D, Cerrano M, Crisà E, et al. How many patients can proceed from chronic myeloid leukaemia diagnosis to deep molecular response and long-lasting imatinib discontinuation? A real life experience. Br J Haematol. 2017;176:669-671.
- Hernández-Boluda JC, Pereira A, Pastor-Galán I, et al; for the Grupo Español de Leucemia Mieloide Crónica (GELMC). Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer J. 2018;8:91.
- Lankford C, Dura J, Tran A, et al. Effect of clinical pharmacist interventions on cost in an integrated health system specialty pharmacy. J Manag Care Spec Pharm. 2021;27:379-384.
- Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320:1889-1898.
- Micromedex RED BOOK. 2022. www.ibm.com/products/micromedex-red-book/pricing. Accessed January 19, 2023.
- Thielen N, van der Holt B, Cornelissen JJ, et al. Imatinib discontinuation in chronic phase myeloid leukaemia patients in sustained complete molecular response: a randomised trial of the Dutch–Belgian Cooperative Trial for Haemato-Oncology (HOVON). Eur J Cancer. 2013;49:3242-3246.
- Rea D, Nicolini FE, Tulliez M, et al; for the France Intergroupe des Leucémies Myéloïdes Chroniques. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129:846-854.
- Horn M, Glauche I, Müller MC, et al. Model-based decision rules reduce the risk of molecular relapse after cessation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Blood. 2013;121:378-384.
- National Cancer Institute SEER Program. Cancer stat facts: chronic myeloid leukemia. https://seer.cancer.gov/statfacts/html/cmyl.html. Accessed August 10, 2022.
- Langabeer SE, Faryal R, O'Dwyer M, Ní Loingsigh S. Patient-initiated discontinuation of tyrosine kinase inhibitor for chronic myeloid leukemia. Case Rep Hematol. 2020;2020:9571691.
- Latremouille-Viau D, Guerin A, Gagnon-Sanschagrin P, et al. Health care resource utilization and costs in patients with chronic myeloid leukemia with better adherence to tyrosine kinase inhibitors and increased molecular monitoring frequency. J Manag Care Spec Pharm. 2017;23:214-224.