Patients with cancer have a 21% increased risk for all-cause in-hospital mortality and rate of acute respiratory failure as a result of COVID-19 compared with patients without cancer.1 A meta-analysis of 38 studies showed that adults with hematologic malignancy and COVID-19 had a 34% increased risk for death, with higher mortality rates (47%) among patients aged ≥60 years.2 COVID-19 vaccines may not be effective in patients with cancer who are currently receiving anticancer treatment because of their reduced innate immune response, impaired, and insufficient activation of antigen-presenting cells, putting them at a higher risk for infection than patients without cancer.3 Thus, alternative modalities of COVID-19 prevention are needed for these patients.
In December 2021, the FDA issued tixagevimab plus cilgavimab an emergency use authorization (EUA) for COVID-19 pre-exposure prophylaxis in select high-risk patients.4 The patients at high risk for COVID-19 include patients aged ≥12 years who weigh ≥40 kg and do not have recent exposure to nor have the coronavirus, and who have an immunocompromised condition that would prevent an adequate immune response to the COVID-19 vaccine, have had a severe adverse reaction to the COVID-19 vaccine, or are unable to receive the COVID-19 vaccine.4 Tixagevimab plus cilgavimab is the first monoclonal antibody for COVID-19 pre-exposure prophylaxis, and it combines the 2 long-acting antibodies of tixagevimab 300 mg and cilgavimab 300 mg. This novel antibody therapy is administered intramuscularly and was initially recommended to be dosed every 6 months for ongoing protection against COVID-19, if needed.4 By simultaneously binding to 2 nonoverlapping sites of the spike protein, tixagevimab plus cilgavimab blocks the interaction between the coronavirus and human angiotensin-converting enzyme 2 receptors on the host cell, thereby neutralizing the virus’s ability to enter the cell and maintaining this protective state for approximately 90 days.5,6
The results from the phase 3 PROVENT trial (NCT04625725) support the initial use of tixagevimab plus cilgavimab in the prevention of COVID-19 in high-risk patients.6 Over a median follow-up period of 83 days, the patients who received tixagevimab plus cilgavimab had a significantly lower incidence of COVID-19 compared with the placebo group (relative risk reduction, 76.7%; 95% confidence interval [CI], 46-90; P<.001). There was no discernible difference in the incidence of adverse events between the 2 groups, and most adverse events were mild or moderate in intensity, including injection-site reactions.6 One of the adverse events that is unique to treatment with tixagevimab plus cilgavimab is anaphylaxis.6 The deaths in the study resulted from COVID-19 infection, myocardial infarction, or renal failure, which were assessed to be unrelated to either treatment arm.6 Based on these trial outcomes and the patient population, the February 2022 National Comprehensive Cancer Network COVID-19 vaccination guide listed tixagevimab plus cilgavimab as a pre-exposure prophylaxis strategy for eligible immunocompromised patients.2,7 Despite these promising findings, only 7% of participants in the PROVENT trial had had or currently have cancer, with 3% of these individuals receiving immunosuppressive therapy at the time of the study’s enrollment.6
Because of the novelty of tixagevimab plus cilgavimab and the lack of real-world data, an interprofessional team of pharmacists, hematologists, and fellow, medical resident, and pharmacy students at the University of Southern California (USC) Norris Comprehensive Cancer Center sought to evaluate the safety and efficacy of tixagevimab plus cilgavimab in the prevention of COVID-19 in patients with cancer. From January 4, 2022, to January 26, 2023, USC Norris administered 839 doses of tixagevimab plus cilgavimab to eligible high-risk patients, with 628 of those doses administered to patients with cancer who were receiving anticancer treatment. Eligible patients were offered treatment with tixagevimab plus cilgavimab per the discretion of their primary hematologist or oncologist and were assessed for appropriateness by the dispensing pharmacist.
Methods
This retrospective study used the TriNetX (clinical data warehouse) query tool to identify patients at Keck Medicine of USC and USC Norris Comprehensive Cancer Center based on the patients’ cancer type. This study was approved by the USC Institutional Review Board.
Adults with cancer were identified using International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes and had to have received tixagevimab plus cilgavimab between January 4, 2022, and December 31, 2022. The control group consisted of patients with the same ICD-9 and ICD-10 codes who did not receive tixagevimab plus cilgavimab within the same time period as the tixagevimab plus cilgavimab group.
The incidence of COVID-19 infection was the primary outcome. If patients were hospitalized for COVID-19, the intensive care unit (ICU) admission, oxygen and ventilator needs, and length of stay were recorded.
The patient characteristics that were collected included age; sex; race and ethnicity; body mass index (BMI); comorbidities; smoking status and/or smoking history (if available); type of malignancy; recent history of hematopoietic stem-cell transplant (HSCT) and/or chimeric antigen receptor (CAR) T-cell therapy within the past 12 months; laboratory results (complete blood count with or without differential); recent history of receiving intravenous immunoglobulin therapy, anti-CD20 monoclonal antibodies, and/or Bruton tyrosine kinase inhibitors within the past 12 months; and COVID-19 vaccination status (including number of doses and manufacturer).
Between January 4, 2021, and December 31, 2022, the patients’ history of COVID-19 was recorded. COVID-19 was confirmed by a positive COVID-19 reverse transcription-polymerase chain reaction test result on chart review. The patients who received tixagevimab plus cilgavimab were followed from the date of the first administration of the combination treatment, whereas the control patients were followed from the first visit in 2022 after January 4, 2022. The patients who did not have COVID-19 after study inclusion were censored at last follow-up or December 31, 2022, whichever occurred earlier. To validate the accurate reporting of positive COVID-19 cases, a survey was sent via email and text to a random selection of untested patients in the tixagevimab plus cilgavimab group. HIPAA and informed consent were obtained by the patient before the inclusion of data in the final analysis.
Statistical Analysis
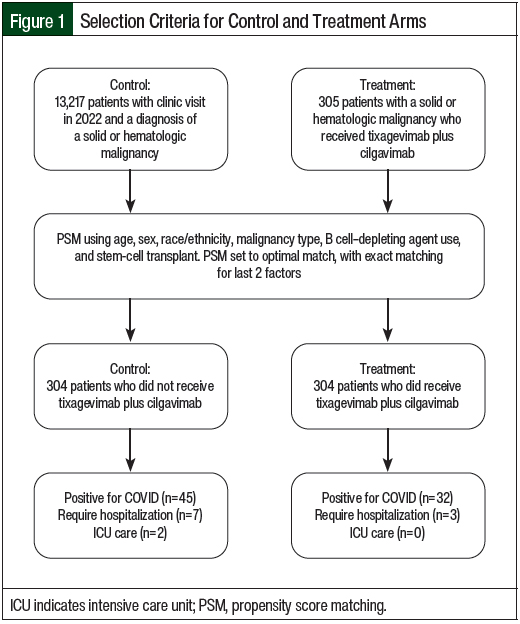
Using a 1:1 ratio for experimental group to controls, the patients were matched according to age, sex, race/ethnicity, malignancy type, B-cell depletion therapy, and history of HSCT. Propensity score matching with optimal pair matching in addition to exact matching for type B cell depletion therapy received and the type of HSCT was implemented (Figure 1). Poisson regression with robust variance and a log of follow-up time as an offset was used to calculate the risk ratio of COVID-19 incidence in patients who received tixagevimab plus cilgavimab relative to propensity score matching controls.
Three models for multivariate analyses were conducted to capture similar models to that of the PROVENT trial, with the additional model to censor the latter half of 2022, because variants were emerging around this time. The descriptive statistics for the patients’ demographics were stratified by tixagevimab plus cilgavimab administration status. The secondary outcomes and descriptive variables were tested using Wilcoxon rank-sum test for numeric variables and Fisher’s exact test for categorical variables. A P value of .05 was considered statistically significant for all analyses conducted in the R Core Team (R) statistical program. The incidence of COVID-19 was estimated using the Kaplan-Meier method stratified by tixagevimab plus cilgavimab administration with days since inclusion as the time scale.
Results
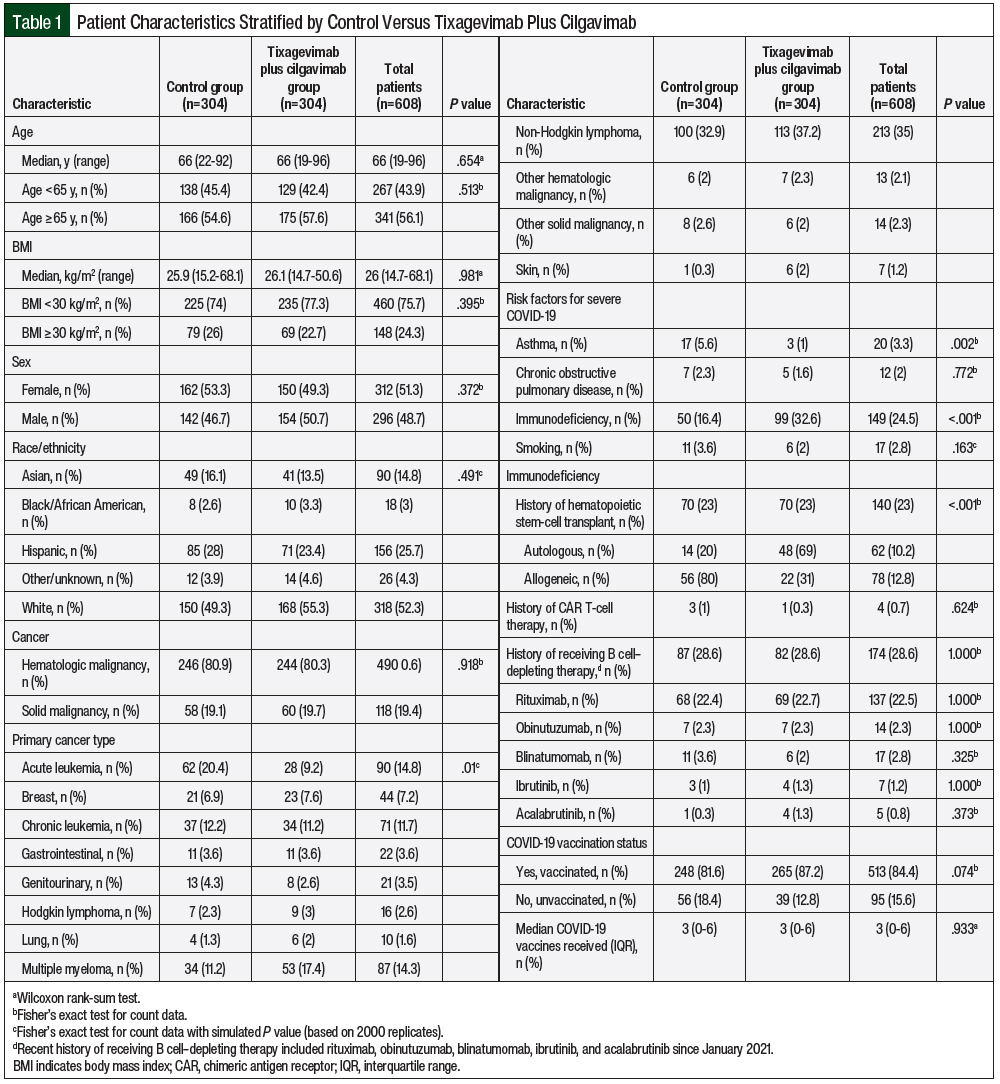
Overall, 13,522 patients with cancer were screened for data analysis in the year 2022, of whom 305 (2.3%) received tixagevimab plus cilgavimab and 13,217 (97.7%) did not receive tixagevimab plus cilgavimab. After propensity score matching, 304 patients who received tixagevimab plus cilgavimab were matched with 304 control patients (Figure 1). The patients’ characteristics after and before matching are shown in Table 1 and Appendix Table S1 (available here), respectively. Before matching, there was a significant difference between the tixagevimab plus cilgavimab group and the control group in age, sex, race/ethnicity, cancer category, and immunodeficiency characteristics (Appendix Table S1). After propensity score matching, all of the variables except the cancer category were no longer significantly different between the groups, including COVID-19 vaccination (Table 1). The individual cancers after propensity score matching are shown in Appendix Table S2 (available here).
The overall patient cohort had a median age of 66 years (range, 19-96 years). The demographic and clinical characteristics were similar at baseline between both of the groups, with the exception of more patients with acute leukemia (9.2% in the tixagevimab plus cilgavimab group vs 20.4% in the control group) and having had allogeneic HSCT (31% in the tixagevimab plus cilgavimab group vs 80% in the control group; P<.001) identified in the control group. The patients’ characteristics are shown in Table 1. There was a difference in the distribution of primary malignancy and immunodeficiency from HSCT between both groups. Most patients in both groups were diagnosed with hematologic malignancies (80.3% in the tixagevimab plus cilgavimab group vs 80.9% in the control group), with non-Hodgkin lymphoma as the most prevalent subtype. Regarding high-risk risk factors for COVID-19, there was a difference in the distribution of asthma and immunodeficiency, but not in chronic obstructive pulmonary disease or smoking.
In each group, 70 (23%) patients had undergone HSCT; however, patients who had had allogeneic HSCT differed significantly between both groups (22 [31%] in the treatment group vs 56 [80%] in the control group). In all, 4 patients received CAR T-cell therapy since January 2021 (1 in the treatment group vs 3 in the control group; P=.624). Rituximab was the most frequently administered B cell–depleting agent between both groups (69 [22.7%] vs 68 [22.4%]; P=1). Regarding COVID-19 vaccination, 513 (84.4%) patients received at least 1 dose of a COVID-19 vaccine (265 [87.2%] in the treatment group vs 248 [81.6%] in the control group; P=.074). A median of 3 COVID-19 vaccine doses were administered between both groups (P=.933). A total of 85 patients who received tixagevimab plus cilgavimab had COVID-19 vaccination after the first dose of tixagevimab plus cilgavimab at a median of 130.5 days, whereas 129 control patients received a vaccine after initial contact in 2022 at a median of 119 days.
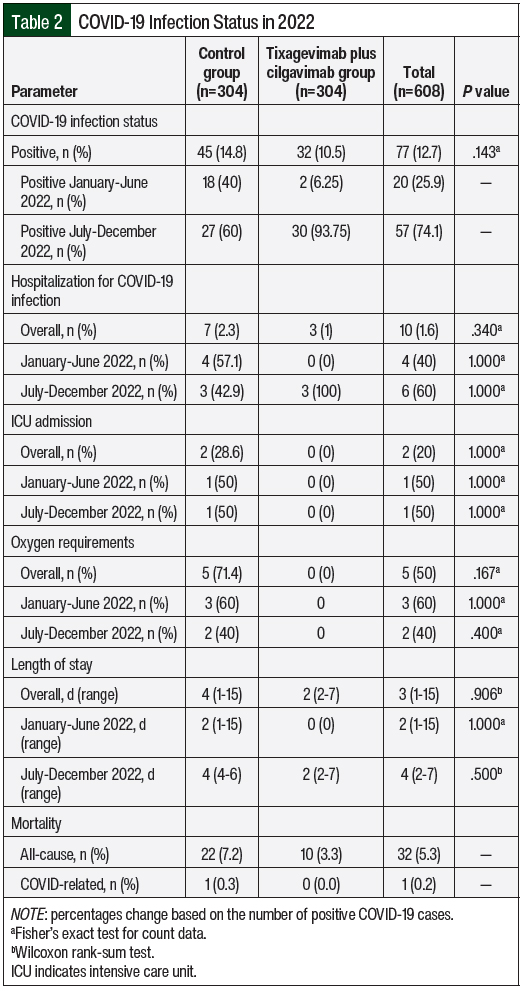
At a median follow-up of 284.5 days (range, 1-361 days) after the administration of tixagevimab plus cilgavimab, 32 (10.5%) patients who received tixagevimab plus cilgavimab tested positive for COVID-19 compared with 45 (14.8%) control patients (Table 2). Between January and June 2022, 2 patients were COVID-19 positive in the tixagevimab plus cilgavimab group versus 18 patients in the control group. The positive cases of COVID-19 among the tixagevimab plus cilgavimab group increased to 30 versus 27 in the control group from July 2022 to December 2022. The median number of days to COVID-19 infection from tixagevimab plus cilgavimab administration was 150.5 days (range, 18-299 days), which reflect less efficacy in the second half of 2022 as new COVID-19 variants emerged.
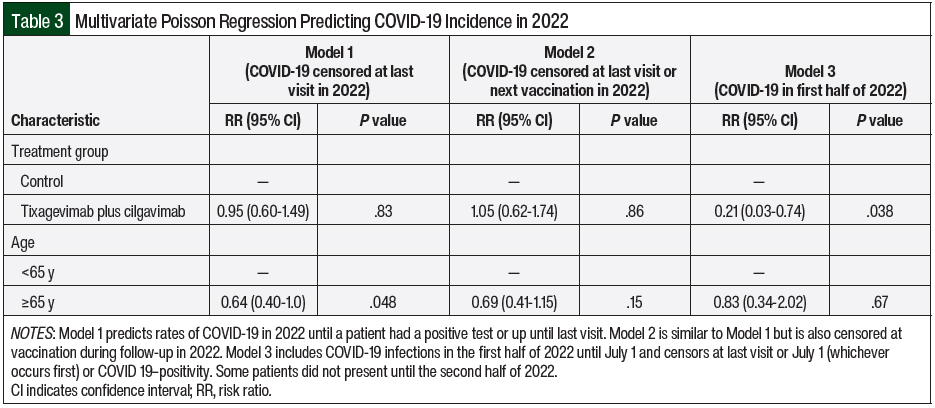
The multivariable models in the entire cohort are shown as 3 models with age included as a covariate (Table 3) based on censoring different time points from the last visit (Model 1), the last visit or next COVID-19 vaccination (Model 2), and COVID-19 positivity in the first half of 2022 (Model 3). When censoring from the last visit or July 1, 2022 (whichever occurred earlier), tixagevimab plus cilgavimab demonstrated a significant reduction in the risk for COVID-19 infection (risk ratio [RR], 0.23; 95% CI, 0.03-0.74; P=.038). However, tixagevimab plus cilgavimab did not demonstrate a reduction in the risk of COVID-19 infection when including the entire year of 2022 (RR, 0.95; 95% CI, 0.60-1.49; P=.83). Censoring the time of vaccination during follow-up in 2022 did not demonstrate a reduction in the risk for COVID-19 either (RR, 1.05; 95% CI, 0.62-1.74; P=.864). In addition, age was borderline significant in Model 1 but was no longer significant when also censoring for vaccinations in Model 2. Fewer patients were included in Model 3 because some patients were not present until the latter half of 2022.
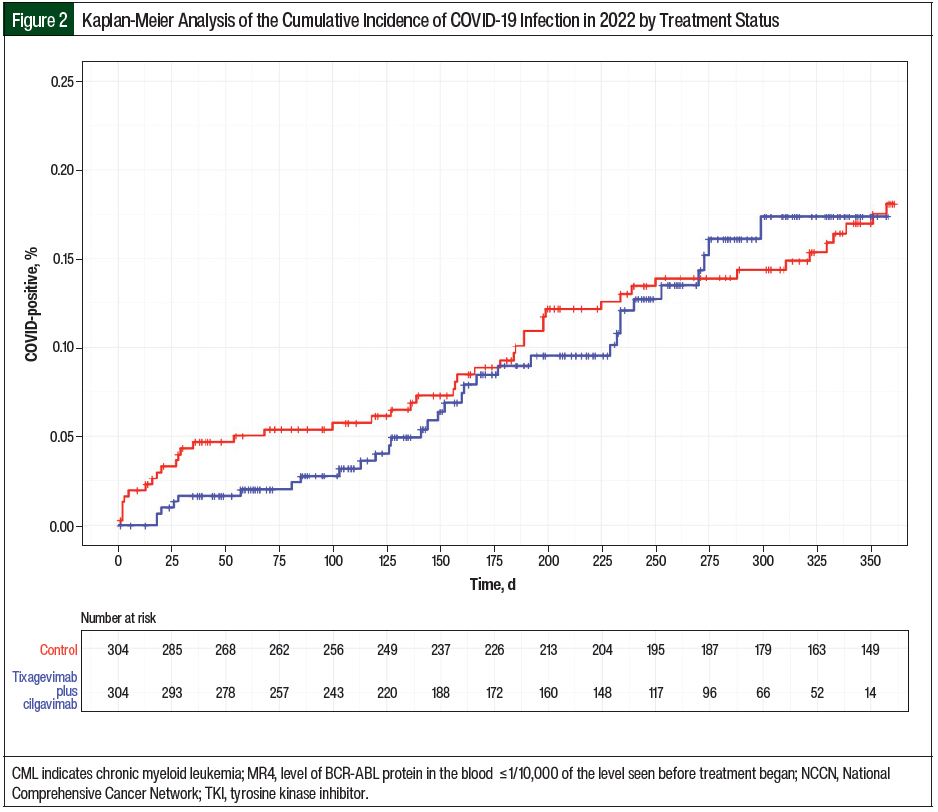
The incidence of COVID-19 infection is estimated using the Kaplan-Meier method stratified by days since inclusion from the administration of tixagevimab plus cilgavimab as the time scale (Figure 2). Although patients had different dates of inclusion from the administration of tixagevimab plus cilgavimab, the median date for the entire cohort was January 29, 2023.
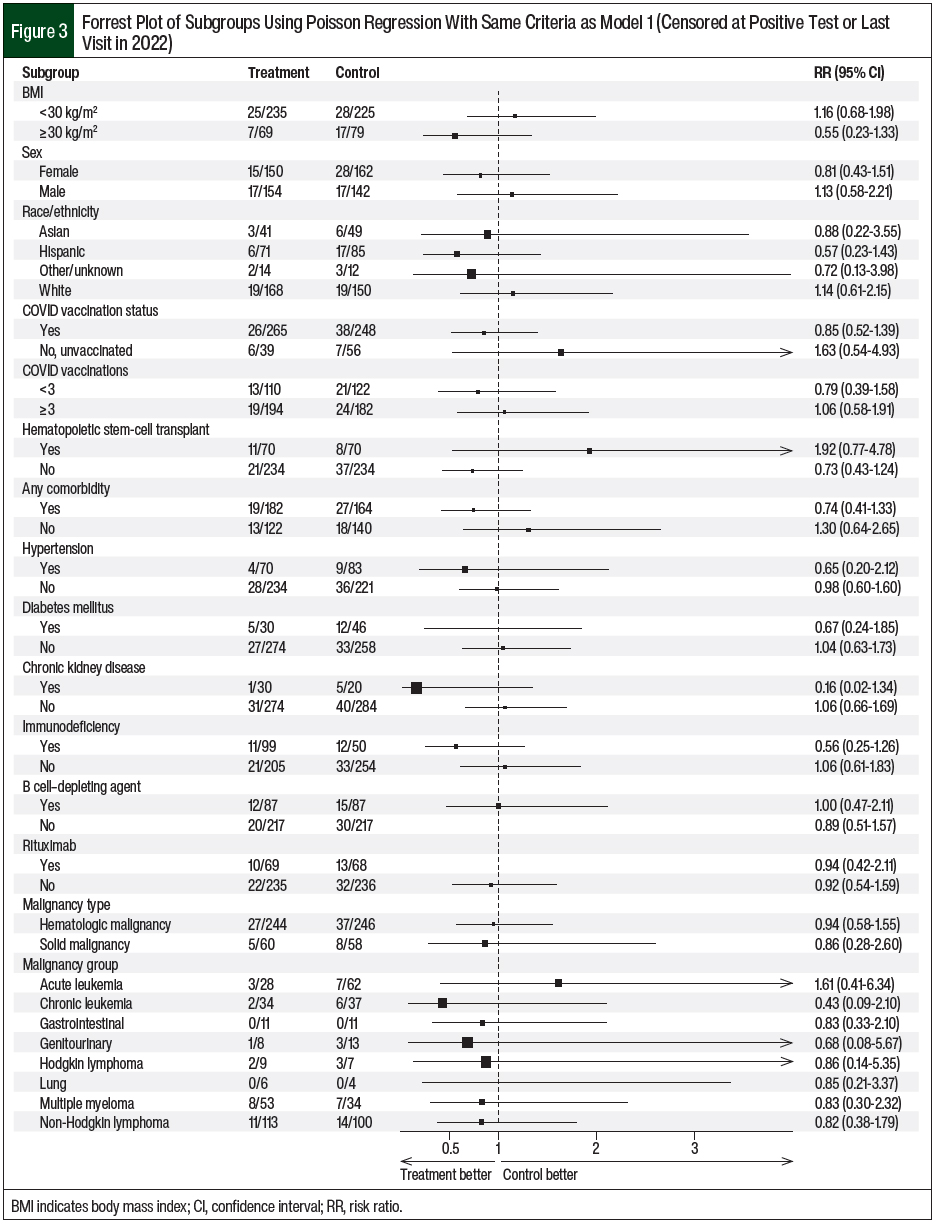
The patients’ characteristics of sex, BMI, and race/ethnicity did not affect the COVID-19 infection risk in patients who received tixagevimab plus cilgavimab compared with the control patients (Figure 3). In addition, COVID-19 vaccination and treatment with tixagevimab plus cilgavimab did not confer a further reduction in COVID-19 infection (RR, 0.85; 95% CI, 0.52-1.39). Subgroup analyses based on comorbidities similarly revealed no reduction in COVID-19 infection for both groups (Figure 3).
Among the patients who tested positive for COVID-19, 3 (1%) patients who received tixagevimab plus cilgavimab were hospitalized compared with 7 (2.3%) patients in the control group (P=.34; Table 2). Between January and June 2022, no patients were hospitalized in the tixagevimab plus cilgavimab group versus 4 patients in the control group. The number of patients hospitalized in the tixagevimab plus cilgavimab group increased to 3, with 3 patients hospitalized in the control group. There was no difference in the severity of hospitalization between both groups in ICU admission (0 [0%] vs 2 [28.6%]; P=.34) and supplemental oxygen requirements (0 [0%] vs 5 [71.4%]; P=.167). The patients in the tixagevimab plus cilgavimab group who were hospitalized did not require ICU admission (P=1) or oxygen (P=.4). Overall, the median length of stay was 2 days (range, 2-7 days) for the tixagevimab plus cilgavimab group compared with 4 days (range, 1-15 days) for the control group (P=.906). When stratified by time, the median length of stay between January and June 2022 was 0 days in the tixagevimab plus cilgavimab group and 2 days (range, 1-15 days) in the control group (P=1). Between July and December 2022, the median length of stay was 2 days (range, 2-7 days) in the tixagevimab plus cilgavimab group and 4 days (range, 4-6 days) in the control group. The all-cause mortality included 10 (3.3%) deaths in the tixagevimab plus cilgavimab group and 22 (7.2%) in the control group. There was only 1 COVID-related death, which was in the control group.
Similar to previous clinical trial data, tixagevimab plus cilgavimab was well-tolerated among the patients, with 5 (1.6%) reports of adverse events and no serious cardiac events.8-10 The adverse events included nausea, vomiting, blurry vision, headache, and fatigue. There were 10 (3.4%) non–COVID-19–related deaths in the tixagevimab plus cilgavimab group and 22 (8.5%) in the control group (P =.48).
A total of 185 patients who received tixagevimab plus cilgavimab and had unconfirmed COVID-19 status based on chart review were randomly surveyed. In all, 36 (19.5%) patients responded, and 3 (8.3%) patients tested positive for COVID-19 in 2022 after receiving their first dose of tixagevimab plus cilgavimab. Two of these COVID-19–positive patients were symptomatic, and none had been hospitalized as a result of COVID-19.
Discussion
In a model adjusted for the timing of COVID-19 infection during the first half of 2022, the patients who received tixagevimab plus cilgavimab had a significant reduction in the risk for COVID-19 infection. Older age had a meaningful impact on the incidence of COVID-19 infections with the emerging Omicron variants, which coincides with previous studies that showed an increased risk for adverse events in older patients.10-16 However, subgroup analyses of other baseline characteristics demonstrated no significant impact on COVID-19 infection (Figure 3). The number of patients in each subgroup may have been too small to detect significance.
The initial 150-mg/150-mg dosing of tixagevimab plus cilgavimab began on January 4, 2022.4 The FDA approved increasing the dose of tixagevimab plus cilgavimab to 300 mg/300 mg on February 24, 2022, because tixagevimab plus cilgavimab was not neutralizing the Omicron subvariants BA.1 and BA.1.1 as effectively as it was neutralizing Omicron subvariant BA.2 at the lower dose.4,9 A total of 109 (35.9%) patients received the initial tixagevimab plus cilgavimab dose before the EUA revision. Among this group, 81 patients completed the full dose of tixagevimab plus cilgavimab (300 mg/300 mg) after the revision and 12 patients received an additional 300-mg/300-mg dose. Although the initial separation of the Kaplan-Meier curves at time zero may not accurately represent the differences in the tixagevimab plus cilgavimab and control groups, this continued separation suggests that tixagevimab plus cilgavimab may have exerted some effectiveness before the prevalence of the Omicron COVID-19 variants. Note that the time zero differed for each patient within the Kaplan-Meier curve.
Beyond day 125, the tixagevimab plus cilgavimab group curve began to approach the control group curve as Omicron subvariants BA.2, BA.2.12.1, BA.4, and BA.5 were circulating in the United States at the time.9 Consequently, the FDA supported redosing every 6 months to maintain the drug concentrations and to confer ongoing protection against coronavirus.4 A total of 86 (28.2%) patients received a supplemental dose after 6 months from their full dose. The patients who received an additional 300-mg/300-mg dose after the initial 150-mg/150-mg dose did not receive a supplemental dose. Moreover, the Kaplan-Meier curves began to converge beyond day 260, and further past day 250, because resistant variants, such as XBB.1.5, accounted for 28% of the circulating variants.4,9
Despite the emerging Omicron COVID-19 subvariants in 2022, it is prudent to consider the real-life differences that were not measurable in this retrospective review. Patients who were considered to have a higher risk for COVID-19 infection because of their underlying cancer tended to receive tixagevimab plus cilgavimab at USC Norris and may take additional safety precautions than less-sick patients with cancer. On the other hand, patients who received tixagevimab plus cilgavimab may have felt protected and may not have taken anti–COVID-19 precautions as stringently as they would without receiving tixagevimab plus cilgavimab. In addition, the Centers for Disease Control and Prevention loosened the COVID-19 restrictions in August 2022, which may coincide with the rise of COVID-19 infections in both groups.
Ongoing phase 3 trials are investigating treatment with tixagevimab plus cilgavimab specifically among immunocompromised patients in the prophylactic setting (SUPERNOVA), as well as among nonhospitalized adults with confirmed COVID-19 infection in the treatment setting (TACKLE).10 The SUPERNOVA trial addresses the gap in the prevention of COVID-19 infection that results from all coronavirus variants among immunocompromised patients with a new formulation of tixagevimab plus cilgavimab. As of January 26, 2023, in vitro laboratory studies with this new formulation demonstrated the neutralization of all coronavirus variants, including variants that are resistant to other monoclonal antibodies.17
Although this initial formulation of tixagevimab plus cilgavimab demonstrated efficacy in the early half of 2022, this advantage was diminished because new COVID-19 variants emerged in the latter half of 2022. The Omicron COVID-19 subvariant, which is closely related to variants that were not neutralized by tixagevimab plus cilgavimab, was estimated to account for 28% of the circulating COVID-19 variants in the United States as of January 6, 2023.9 Additional in vitro and in vivo data confirmed that tixagevimab plus cilgavimab lacked activity against the more predominant coronavirus variants, which led to the removal of EUA status from tixagevimab plus cilgavimab on January 26, 2023.9 When stratified by time, none of the patients who received tixagevimab plus cilgavimab were hospitalized for or died as a result of COVID-19 infection between January and June 2022. Although the incidence of hospitalization increased between July and December 2022 for patients who received tixagevimab plus cilgavimab, these patients did not require ICU admission or supplemental oxygen and had a shorter hospital length of stay than the control group. However, these findings were low in incidence and lacked statistical significance. Interestingly, the length of stay was shorter in the control group when comparing the first and second halves of 2022. Newer variants in 2022 may have been associated with less severe disease, leading to less hospitalization for even high-risk patients who did not receive any pre-exposure prophylaxis. Considering the elevated disease burden and financial strain faced by patients with cancer, it is essential that pharmacists carefully assess the value of new COVID-19 therapies for vulnerable patients based on patient-specific factors that would confer a higher risk of COVID-19 infection. Further studies could explore treatment with tixagevimab plus cilgavimab in the post–COVID-19 exposure setting, because the TACKLE study included a larger number of patients with cancer than our study.
Limitations
The limitations of this review include the lack of events in smaller subgroups, and this study could not estimate efficacy. Unlike previous reviews of tixagevimab plus cilgavimab in a similar patient population, no additional baseline or coexisting conditions significantly impacted the incidence of COVID-19 infection based on subgroup analysis.6,10-12 This study was also limited by the significant difference in the number of patients with acute leukemia and a history of allogeneic HSCT between both groups. These patient subgroups in particular have a higher risk for neutropenia or lymphopenia and may have received myeloablative chemotherapy, relative to patients with other malignancies. Thus, the findings from this study may be skewed because the control group may have had a higher baseline risk for COVID-19 as a result of having more severe immunosuppression. This study was limited in its ability to pull data from the clinical data warehouse specifically for the type of HSCT. In addition, there is systematic bias from variability in the reporting of COVID-19 infection, the low number of survey responses, and in the inability to capture survey responses from the control group.
Currently, reporting positive COVID-19 cases within the electronic medical record is not standardized. The low response rate of the randomly surveyed treatment group limits understanding the true rate of positive cases. The absence of surveying the control group further underestimates the amount of positive COVID-19 cases and may skew the final number of positive cases toward the treatment group. A standardized reporting process or more robust survey model should be considered to best capture breakthrough cases of COVID-19. Of note, this retrospective review only captured patients within 2022 and did not include any patients who received tixagevimab plus cilgavimab in 2023. Last, the therapeutic class and timing of anticancer therapy around the time of COVID-19 infection, as well as stage of diagnosis, could have been additional contributors. However, the connection between anticancer therapy and COVID-19 has been inconsistently demonstrated in previous studies.11-16 In a large National COVID Cohort Collaborative study, receiving chemotherapy within 30 days of COVID-19 infection increased the mortality risk, but not among patients who received immunotherapy or targeted therapy.16 Future evaluations of an improved formulation of tixagevimab plus cilgavimab could consider the implications of more than immunosuppression from ongoing anticancer therapy.
Notable strengths for this single-center study include its diverse sample size to evaluate the implications of several characteristics. Both groups of patients were similar in age, sex, and comorbidities. A majority of patients were aged >60 years and were diverse in race and ethnicity, reflective of the Greater Los Angeles population. Similar to the PROVENT trial, this study also included populations that were disproportionately affected by COVID-19, including Black and Hispanic or Latinx populations. Although limited in design, the survey was intended to capture undocumented COVID-19 cases that may have otherwise been overlooked as a result of possible milder disease, lack of symptoms, or lack of testing.
Conclusion
Although this study did not show a significant reduction in COVID-19 infection with tixagevimab plus cilgavimab, this drug combination may offer clinically meaningful reductions in COVID-19 infections and related complications in early 2022 for patients who have a lower risk for immunosuppression from their underlying cancer and anticancer treatment. The diminished efficacy may be attributable to the high mutation rates of the coronavirus and the medication’s inability to adapt to the newer strains. This retrospective study provides insight into alternative approaches, including pre-exposure prophylaxis, to minimize the complications of COVID-19 infection among vulnerable populations. Further studies of how to target regions of the virus that have not mutated are needed to create a prophylactic drug that will be able to protect our immunocompromised population.
Throughout the pandemic, pharmacists have played a pivotal role in the battle against COVID-19 by advocating for and administering vaccines and dispensing antiviral medications to minimize the severity of and treat COVID-19. The careful assessment of monoclonal antibody therapies is another way clinical pharmacists have demonstrated their commitment to ensuring optimal outcomes in high-risk patients with COVID-19.
Acknowledgments
We would like to acknowledge Ryan Lee, MPH, and Viviana Rodriguez, BA Psychology, who conducted the surveys.
Funding Source
Funding for this study was provided by USC Keck Medicine.
Author Disclosure Statement
Dr Mohrbacher provides consulting to Bristol Myers Squibb (no conflict of interest with Evusheld); Dr Tong, Dr Ashouri, Mr Cai, Dr Fernandez Hernandez, Dr Shi, Dr Yaghmour, and Dr Ali have no conflicts of interest to report.
References
- Abuhelwa Z, Alsughayer A, Abuhelwa AY. In-hospital mortality and morbidity in cancer patients with COVID-19: a nationwide analysis from the United States. Cancers (Basel). 2022;15(1):222.
- Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881-2892.
- Negahdaripour M, Shafiekhani M, Moezzi SMI, et al. Administration of COVID-19 vaccines in immunocompromised patients. Int Immunopharmacol. 2021;99:108021.
- Fact sheet for healthcare providers: Emergency Use Authorization for Evusheld (tixagevimab co-packaged with cilgavimab). Evusheld (tixagevimab) injection; (cilgavimab) injection, co-packaged for intramuscular use [emergency use authorization]. AstraZeneca; January 2023. www.fda.gov/media/154701/download Accessed February 24, 2023.
- Loo YM, McTamney PM, Arends RH, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med. 2022;14:eabl8124. Erratum in: Sci Transl Med. 2022;14:eabg8900.
- Levin MJ, Ustianowski A, De Wit S, et al; for the PROVENT study group. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188-2200.
- National Comprehensive Cancer Network. Recommendations of the National Comprehensive Cancer Network (NCCN) Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis. Version 5.0. January 4, 2022. Accessed February 24, 2023.
- Nguyen Y, Flahault A, Chavarot N, et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect. 2022;28:1654.e1-1654.e4. S1198-743X(22)00383-4. Epub ahead of print.
- US Food & Drug Administration. FDA announces Evusheld is not currently authorized for emergency use in the U.S. January 26, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us. Accessed February 24, 2023.
- Montgomery H, Hobbs FDR, Padilla F, et al; for the TACKLE study group. Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022;10:985-996.
- Chavez-MacGregor M, Lei X, Zhao H, et al. Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. JAMA Oncol. 2022;8:69-78.
- Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19–infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113:371-380. Erratum in: J Natl Cancer Inst. 2022;114:328-330.
- Kuderer NM, Choueiri TK, Shah DP, et al; for the COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907-1918. Erratum in: Lancet. 2020;396:758.
- Lee LYW, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919-1926. Erratum in: Lancet. 2020;396:534.
- Newman LA, Winn RA, Carethers JM. Similarities in risk for COVID-19 and cancer disparities. Clin Cancer Res. 2021;27:24-27.
- Sharafeldin N, Bates B, Song Q, et al; for the National COVID Cohort Collaborative. Outcomes of COVID-19 in patients with cancer: report from the National COVID Cohort Collaborative (N3C). J Clin Oncol. 2021;39:2232-2246.
- Phase III Double-Blind, Placebo-Controlled Study of AZD7442 for Post-Exposure Prophylaxis of COVID-19 in Adults (STORM CHASER). NLM identifier: NCT04625972. Updated October 25, 2022. https://clinicaltrials.gov/ct2/show/NCT04625972. Accessed February 25, 2023.