Monoclonal antibodies have been associated with nonallergic infusion reactions mediated by a cytokine release syndrome (CRS).1 When the monoclonal antibody binds to the antigen of the target cell, specialized cytokines recruit immune-effector cells and complement molecules. As a result, this target cell is tagged and destroyed. Furthermore, these target cells and immune-effector cells release cytokines into circulation that can cause infusion-related reactions. The symptoms of CRS are generally mild to moderate in severity and typically occur within the first 2 hours of administration of an infused monoclonal antibody.1 The symptoms are relieved with stopping or slowing the rate of infusion, and they most often occur with the first dose of an infusion, when the tumor burden is highest (ie, more targeted cells exist). The symptoms of CRS typically subside with each subsequent dose of a monoclonal antibody.1
As with other drugs, such as carboplatin, oxaliplatin, and L-asparaginase, monoclonal antibodies carry a risk for hypersensitivity reactions.1 Hypersensitivity reactions can occur at any time, are allergic in nature, and can occur with any drug. The symptoms of hypersensitivity include hypotension, shortness of breath, flushing, chest pain, and throat swelling.1 Intervention with medications, such as steroids, diphenhydramine, and fluids, is necessary in addition to stopping the infusion. Because hypersensitivity reactions are rare and are not as predictable as infusion-related reactions, observation times do not mitigate the risk for hypersensitivity.1
Pertuzumab is a humanized anti-HER2/neu receptor monoclonal antibody used in combination with trastuzumab and a taxane (paclitaxel or docetaxel) for the treatment of HER2-positive metastatic breast cancer in patients who have not received anti-HER2 therapy or chemotherapy for metastatic disease.2,3 Pertuzumab is also indicated in combination with trastuzumab and chemotherapy for the neoadjuvant treatment of HER2-positive, locally advanced, inflammatory, or early-stage breast cancer, as well as for the adjuvant treatment of HER2-positive early breast cancer that has a high risk for recurrence.2,3
According to pertuzumab’s prescribing information, in the CLEOPATRA study, patients who received pertuzumab had a rate of infusion-related reactions of 13% compared with 9.8% in patients who received placebo.3 In the APHINITY trial, 21% of the patients who received pertuzumab had an infusion-related reaction compared with 18% of patients in the placebo group.3 By subtracting the rate of infusion-related reactions in the group that received pertuzumab from the rate in the group that received placebo, the rate of all-grade pertuzumab infusion-related reactions is approximately 3%. The rate of grade 3 or 4 infusion-related reactions was <1% in the CLEOPATRA trial. In the APHINITY trial, the rate of grade 3 or 4 infusion-related reactions was 1% in the patients who received pertuzumab compared with 0.7% in the placebo arm.3 Because of the low rate of infusion-related reactions, these data were not published in the CLEOPATRA or APHINITY trials.4,5 The most common (≥1%) infusion-related reactions with pertuzumab include pyrexia, chills, fatigue, headache, asthenia, and vomiting.3 Because of these infusion-related reactions, the prescribing information for pertuzumab states that patients must be observed for a period of 60 minutes after the first infusion of pertuzumab and for 30 minutes after subsequent infusions.3
Ado-trastuzumab emtansine is a HER2-targeted antibody and microtubule inhibitor conjugate indicated, as a single agent, for the treatment of HER2-positive, metastatic breast cancer in patients who previously received trastuzumab and a taxane separately or in combination.6 In addition, ado-trastuzumab emtansine is approved for the adjuvant treatment of HER2-positive early breast cancer in patients who have residual invasive disease after receiving neoadjuvant therapy with a taxane and trastuzumab-based treatment.6 According to ado-trastuzumab emtansine’s prescribing information, the incidence of infusion-related reactions was 1.4% in the ado-trastuzumab emtansine treatment group, with no grade 3 or 4 infusion-related reactions.6 Because of the low rate of infusion-related reactions, these rates were not included in the data for the EMILIA trial.7 Because of the infusion-related reactions, the prescribing information for ado-trastuzumab emtansine states that patients must be observed for a period of 90 minutes after the first infusion of ado-trastuzumab emtansine and for 30 minutes after subsequent infusions.6
According to personal communication in 2015 with Amy Andriola, a medical science liaison at Genentech, the protocol for the approval studies for pertuzumab and ado-trastuzumab emtansine contained the observation times to mitigate the risk for the infusion-related reactions that are associated with monoclonal antibodies. The observation times were then included in the prescribing information for pertuzumab and ado-trastuzumab emtansine based on the design of the clinical studies for the drugs’ approvals.3,6
The observation times after patients have received pertuzumab or ado-trastuzumab emtansine are not based on any data that exist specifically for these drugs. The same risk for infusion-related reactions exists for other monoclonal antibodies, such as trastuzumab, a monoclonal antibody targeted against HER2. With ≥3 doses of trastuzumab, the estimated risk for an infusion reaction is approximately 4%.8
There is no specific amount of observation time required after patients receive an infusion of trastuzumab. The standard of care for trastuzumab infusion is to discharge patients from the clinic immediately after the infusion.
The goal of this study was to provide data on the rate of infusion-related reactions while receiving pertuzumab or ado-trastuzumab emtansine and to determine the observation time for patients after receiving pertuzumab or ado-trastuzumab emtansine.
The primary outcome of this study was to determine the proportion of patients who have a reaction after receiving the third dose of pertuzumab or ado-trastuzumab emtansine in patients who did not have a reaction after receiving the first 2 doses of these drugs. As noted previously, infusion-related reactions have the highest probability of occurence with the first dose of pertuzumab or ado-trastuzumab emtansine, which decreases with each subsequent dose of these drugs. We chose to evaluate the third dose of pertuzumab or ado-trastuzumab emtansine because most infusion-related reactions will occur after the patient has received the first 2 doses.
Methods
This retrospective chart review included patients in the M Health Fairview system who received pertuzumab or ado-trastuzumab emtansine infusions. The reactions during the infusion and within the observation time for the drug were recorded and the incidence was calculated. The symptoms of infusion-related reaction included fever, chills, fatigue (in combination with another symptom listed), headache, extreme physical weakness, rash, vomiting, muscle pain, and abnormal taste sensitivity.
The patients had to receive pertuzumab or ado-trastuzumab emtansine infusions at an M Health Fairview oncology infusion center, which included M Health Fairview Maple Grove Cancer Center, Fairview Southdale Oncology Center, Fairview Ridges Specialty Care Center, and M Health Fairview Clinics and Surgery Center. The patient selection criteria included age >18 years, having a diagnosis of breast cancer (metastatic or neoadjuvant), and having an electronic medical record that indicates approval to be used for research purposes. Patients were excluded if their diagnosis was for another oncologic indication, such as HER2-positive gastric cancer.
An analysis of the data was performed using descriptive statistics. For the primary aim, point estimates and exact 95% confidence intervals (CIs) for the proportion of patients who have any infusion-related reactions were calculated. Infusion-related reactions at subsequent infusions are also described, although they were not included in the primary outcome because they were not independent observations.
The study received approval from the Masonic Cancer Center Cancer Protocol Review Committee and the institutional review board.
Results
Pertuzumab
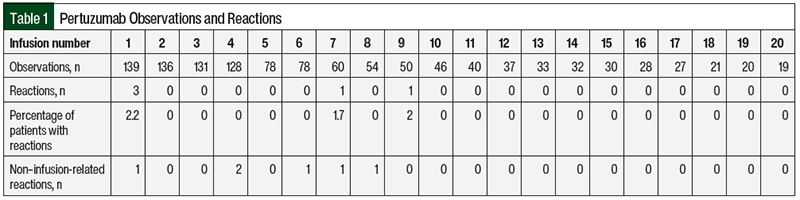
A retrospective review of patient data from October 2011 to May 2017 yielded a total of 144 patients for possible inclusion in the pertuzumab arm of the study. Of the 144 patients, 16 patients did not meet the study inclusion criteria because they did not receive the first 3 doses of pertuzumab within the M Health Fairview system. Although their infusions are included in Table 1, they were not part of the primary analysis. Table 1 summarizes the number of reactions by infusion number for all 144 patients.
Our prespecified primary outcome was the percentage of reactions during and after receiving the third infusion of pertuzumab for patients who did not have a reaction after receiving infusions 1 and 2. Of the 128 patients, 3 patients had an infusion-related reaction during the first or second dose of pertuzumab, leaving 125 patients for analysis. No reactions were observed in the 125 patients who did not have a reaction after previously receiving an infusion of pertuzumab (95% CI, 0%-2.9%). Table 1 includes all observations, not only patients who did not have a reaction after receiving a previous infusion of pertuzumab.
A total of 3 patients had infusion-related reactions during the first infusion of pertuzumab, which included rash and chills. The infusion was stopped and the patients received steroids and diphenhydramine. All 3 of these patients subsequently received at least 10 more doses of pertuzumab without incident. One patient had grade 1 muscle pain during her seventh infusion. No intervention was necessary, and the patient received another 4 doses of pertuzumab without incident.
The only grade 3 reaction was a rash in 1 patient during her ninth infusion of pertuzumab. She did not receive her first 2 doses in the M Health Fairview system. She received her third through eighth doses of pertuzumab without incident. The patient received steroids and diphenhydramine and was sent to the emergency department for further treatment. This patient subsequently received another 13 doses of pertuzumab without incident.
Some patients did have hypersensitivity reactions with pertuzumab treatment. One patient had a cough while receiving the fourth dose of pertuzumab. The infusion was stopped, and methylprednisolone was given. The infusion was then restarted and finished without incident. On receiving doses 6, 7, and 8 of pertuzumab, the same patient had more severe hypersensitivity reactions, including shortness of breath, chest tightness, and flushing during the infusions. Ultimately, treatment with pertuzumab was discontinued in this patient after attempting infusion of the eighth dose. The patient received steroids and diphenhydramine, and no emergency services were necessary.
A second patient also had hypersensitivity reactions while receiving the fourth dose of pertuzumab, including chest pain, shortness of breath, flushing, nausea, and tingling in the arms and hands. Treatments with famotidine and lorazepam were administered, but no emergency services were necessary. Treatment with pertuzumab was permanently discontinued in this patient.
A third patient had sharp chest pain for approximately 2 to 3 seconds during the first infusion of pertuzumab. The infusion was stopped and then restarted after the chest pain resolved. No intervention was necessary, and the patient received another 3 doses of pertuzumab without incident.
Ado-Trastuzumab Emtansine
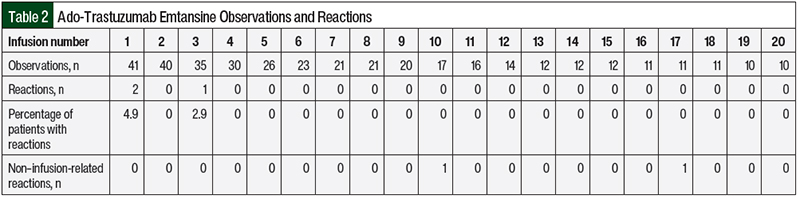
A retrospective review of patient data from October 2011 to May 2017 yielded a total of 43 patients for possible inclusion in the ado-trastuzumab emtansine arm of the study. Of these 43 patients, 8 patients did not receive the first 3 doses of ado-trastuzumab emtansine within the M Health Fairview system, so they did not meet the study inclusion criteria. Their infusions are still included in Table 2, but they were not part of the primary analysis. Table 2 summarizes the observations for ado-trastuzumab emtansine infusion for all 43 patients.
Table 2 summarizes the number of reactions to treatment with ado-trastuzumab emtansine by infusion number. Of the 35 patients who received ado-trastuzumab emtansine, 2 patients had an infusion-related reaction while receiving the first dose. Both patients received ≥7 subsequent infusions of ado-trastuzumab emtansine without having a reaction. Our prespecified primary outcome was the percentage of reactions after the third infusion of ado-trastuzumab emtansine in patients who did not have a reaction while receiving the first and second infusions of the drug. In the 33 patients who had no previous reaction after receiving ado-trastuzumab emtansine, there was only 1 (3%) reaction, which was a grade 1 rash during the third infusion that did not require any medical or pharmaceutical intervention (95% CI, 0%-15.3%). No grade 3 or 4 infusion-related reactions occurred while receiving treatment with ado-trastuzumab emtansine.
There were 2 non–infusion-related reactions while receiving treatment with ado-trastuzumab emtansine. One patient had shortness of breath as a result of a pleural effusion before the start of treatment with ado-trastuzumab emtansine. During the infusion, the patient had chest tightness and worsened shortness of breath. The infusion was stopped and methylprednisolone was given. The infusion was not restarted. This patient received another 13 infusions of ado-trastuzumab emtansine without incident. A second patient had a grade 3 headache that started before the infusion of ado-trastuzumab emtansine, which improved after the patient received acetaminophen. The infusion of ado-trastuzumab emtansine was administered without interruption and no other interventions were required.
Discussion
Pertuzumab and ado-trastuzumab emtansine have a recommended observation time of 30 minutes after the infusion of the first dose. There are no data that directly support this required observation time, however. Furthermore, other monoclonal antibodies that carry the same risk for infusion-related reactions, such as trastuzumab, do not have this recommended observation time. This observation time takes up resources within the infusion center, including chair time, that could be used for another patient.
In patients who are receiving ado-trastuzumab emtansine, we eliminated the observation time if the patient received the first 2 doses and did not have any reaction.
In patients who are receiving pertuzumab, we eliminated the observation period after treatment with pertuzumab for all doses. M Health Fairview’s protocol was to administer pertuzumab first, followed by trastuzumab; therefore, the risk for eliminating the observation time after pertuzumab is further reduced because the patient is being monitored while receiving trastuzumab. It is worth noting that if a patient were to have a reaction while receiving trastuzumab, it could potentially be from either drug. Given the exceptionally low rate of having a reaction after receiving pertuzumab, this risk does not warrant having an observation time for pertuzumab before administering trastuzumab.
This retrospective review provides data that show the relatively low risk for infusion-related reactions, particularly severe infusion-related reactions. No reactions were noted during the observation time for any of the 1187 infusions of pertuzumab and 393 infusions of ado-trastuzumab emtansine. All of the reactions occurred during the infusion. These data justify a prospective trial to evaluate the need for observation times after treatment with pertuzumab and ado-trastuzumab emtansine.
In addition, although it is reasonable to assume that other institutions have also eliminated observation times for pertuzumab and ado-trastuzumab emtansine, we do not have data from these institutions.
Limitations
This study has limitations. The small size of this study is a major limitation. Although this study provides data from 6 years, the sample size of patients who received ado-trastuzumab emtansine was small. Because the rate of infusion-related reactions while receiving ado-trastuzumab emtansine was only 1.4%, a larger sample size is needed to confirm these results.
Another limitation is the reliability of the documentation by the nursing staff. Nurses are required to document every infusion, but the system did not have standards for how much detail should be documented for a reaction.
Conclusion
Based on these data, we eliminated the observation time for all doses of pertuzumab. Pertuzumab is always given with trastuzumab. Because our protocols are built to administer pertuzumab first, followed by trastuzumab, the risk for infusion-related reactions is further reduced because we are observing the patients during their trastuzumab infusion. For ado-trastuzumab emtansine, we eliminated the observation time if the patient received the first 2 doses and did not have any reaction.
Author Disclosure Statement
Dr Smith is on the Speaker’s Bureau of Karyopharm.
References
- Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs. 2010;14(2):E10-E21.
- National Guidelines Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast cancer. Version 4.2023. March 23, 2023. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 3, 2023.
- Perjeta (pertuzumab) injection, for intravenous use [prescribing information]. Genentech; February 2021. www.gene.com/download/pdf/perjeta_prescribing.pdf. Accessed April 24, 2023.
- Smyth LM, Iyengar NM, Chen MF, et al. Weekly paclitaxel with trastuzumab and pertuzumab in patients with HER2-overexpressing metastatic breast cancer: overall survival and updated progression-free survival results from a phase II study. Breast Cancer Res Treat. 2016;158:91-97.
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122-131.
- Kadcyla (ado-trastuzumab emtansine) for injection, for intravenous use [prescribing information]. Genentech; February 2022. www.gene.com/download/pdf/kadcyla_prescribing.pdf. Accessed April 24, 2023.
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783-1791.
- Smith IE. Efficacy and safety of Herceptin in women with metastatic breast cancer: results from pivotal clinical studies. Anticancer Drugs. 2001;12(suppl 4):S3-S10.