Hematologic malignancies are a heterogenous group of cancers that originate from the uncontrolled proliferation of hematopoietic and lymphoid tissues and give rise to leukemia, lymphoma, or myeloma. Over the past 20 years, the incidence of hematologic malignancies has increased dramatically.1 This rapid increase is strongly correlated with the aging population of baby boomers (those born 1946-1964) because there is a projected 67% increase in the overall incidence of cancer by 2030 among older (aged ≥65 years) adults.1,2 In response to the emergent need for available therapies, oral oncolytics have been increasingly studied for the treatment of hematologic malignancies, leading to a substantial rise in the approval of oncolytic drugs over the past several years.3 Some oral oncolytics for the management of hematologic malignancies have unique pharmacologic properties, dosing, and side-effect profiles, which can present challenges with incorrect dosing, limited drug monitoring, and drug-drug or drug-food interactions, which, if not properly addressed, can lead to serious adverse events, morbidity, and mortality.4,5
Oral chemotherapy offers several advantages over parenteral drugs, including convenience for patients, flexibility in timing, and location of administration. An additional advantage, which prevented a lapse in dosing during the COVID-19 pandemic, is a reduced burden of clinic visits at a cancer center. Finally, oral oncolytic therapy options may minimize or eliminate infusion center visits for injectable treatments, granting patients a greater sense of normalcy by allowing more time to carry out activities of daily living.
A major disadvantage of oral oncolytics is financial toxicity. Financial toxicity refers to the direct and indirect costs that lead to a negative patient-level impact resulting from a cancer diagnosis.6 Unfortunately, despite insurance approval of a drug, patients may still have prohibitive out-of-pocket costs that can contribute to nonadherence. Patient adherence is defined as the intake of more than 80% of the prescribed medication.7 Aside from cost, patient adherence may also be suboptimal in patients with drug-related adverse events. Pharmacists employed in oncology clinics and specialty pharmacies are often responsible for managing drug-related adverse events, continuously providing patient education, and managing medication access issues.
Despite the convenience of oral oncolytic drugs, patient adherence and the identification and management of treatment nonresponse and/or adverse events remain major challenges.8-11 Many oral oncolytics, such as tyrosine kinase inhibitors, immunomodulatory agents, proteasome inhibitors, phosphatidylinositol 3-kinase inhibitors, and Bruton tyrosine kinase inhibitors, are effective therapeutic options. However, factors such as drug resistance, tolerability by patients, and the successful induction of durable responses may make patients prefer one oral oncolytic drug versus another.12,13
With the rising number of oral oncolytics, the oncology pharmacy specialist’s role in the outpatient clinic setting has expanded to include monitoring medication therapy, managing adverse reactions to drugs, identifying preventable medication errors, optimizing therapy, assessing patients to improve quality of life, and re-establishing care for patients who were previously lost to follow-up.14-16
The objective of this study was to describe the clinical role of oncology pharmacy specialist–driven interventions in patients who are receiving oral oncolytics that were prescribed in a hematology clinic at a community cancer center.
Methods
This retrospective chart review included patients who initiated treatment with or who were already receiving oral oncolytics between January 1, 2021, and June 30, 2021, at Miami Cancer Institute Baptist Health South Florida (MCI). Patients were included in the study if they were aged ≥18 years, had a confirmed hematologic malignancy, and were receiving oral oncolytic therapy. Patients who received an oral oncolytic drug as part of their overall treatment plan were also included.
The primary outcome of the study was to describe the type and frequency of oncology pharmacy specialist–driven interventions in the management and safety of oral oncolytics. The secondary outcome was to identify the trends between the number and type of interventions and the baseline patient characteristics and drug class. Descriptive statistics were used in the data analysis. This study excluded patients who were enrolled in clinical trials, had interventions unrelated to the management of oral oncolytic therapy, and had supportive care interventions that were unrelated to oral oncolytic therapy. Patients who were enrolled in a clinical trial were excluded from the study because they were managed according to specific study protocols that were not addressed in this study. At our institution, oncology pharmacy specialists intervene on intravenous chemotherapy and related supportive care; thus, those interventions that did not relate to oral oncolytic therapy were excluded from review in this study.
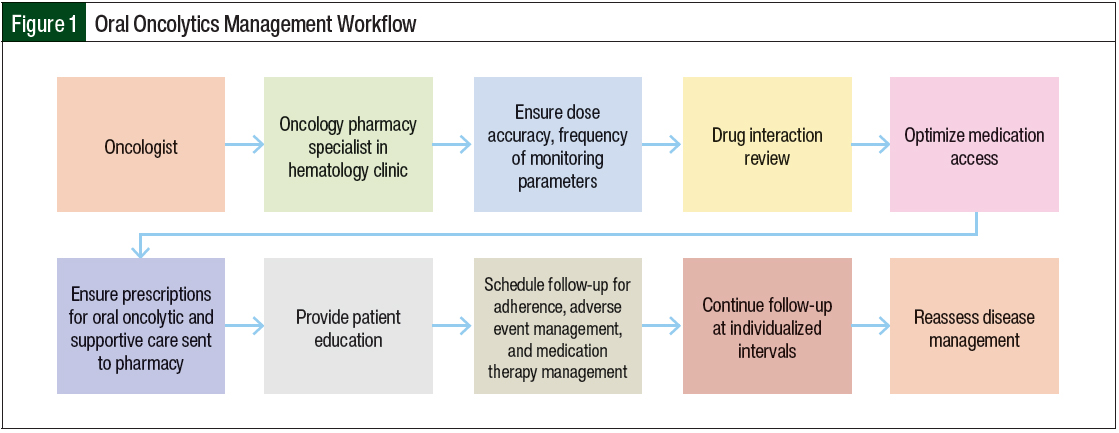
The oral oncolytic management workflow at MCI starts when an oncologist in the hematology clinic prescribes an oral oncolytic. From there, the oncology pharmacy specialist ensures the dose accuracy, advises on the frequency of the monitoring parameters, performs drug interaction review, assists with medication access, performs patient education, schedules follow-up visits for adherence, manages the patient’s adverse events, and performs medication therapy management (Figure 1). MCI has an embedded specialty pharmacy on-site that is available to receive prescriptions. Once an order is routed to the embedded specialty pharmacy, a team of dedicated personnel facilitate the process of insurance benefits investigation and prior authorization. The oncology pharmacy specialists at MCI work closely with the specialty pharmacy on-site as well as external specialty pharmacies to address any issues relating to authorization or co-pay affordability before the medication dispensation.
Patient adherence at MCI is monitored by surveying the patient at each reassessment session performed by the oncology pharmacy specialist. The survey assesses the number of pills missed in the past 7 days and any barriers to adherence. Once the oncology pharmacy specialist identifies the barriers, he or she provides solutions to minimize financial toxicity and improve adherence, such as the use of a calendar, alarm clocks, or phone applications.
Medication therapy management services include medication reconciliation, the identification of unnecessary therapy, assessing for drug compliance, assessing drug interactions, and managing drug-related adverse events. Medication therapy management interventions at MCI include a review of patients’ comorbidities and corresponding medications that could potentially affect the success of the oral oncolytic drug intake, such as hypertension, atrial fibrillation, and hypercoagulable state. Patients with multiple comorbidities are at risk for adverse reactions and drug interactions and might have altered pharmacokinetics.
Descriptive statistical methods (frequency, median, and range) were used for the analysis of the primary and secondary end points. This study was approved by the MCI and Baptist Health South Florida Institutional Review Board.
Results
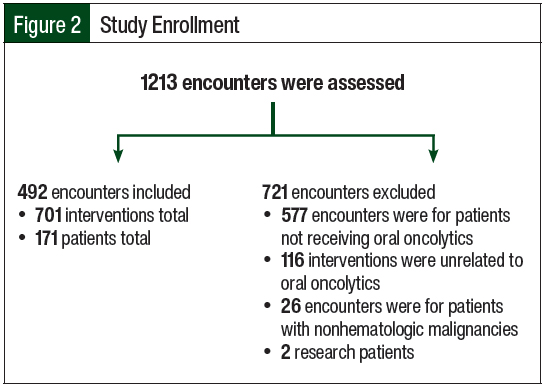
Among the 1213 encounters that were evaluated, 492 encounters were included. Every encounter included at least 1 intervention, with 701 interventions evaluated in a total of 171 patients. The exclusion of the remaining patients and encounters was primarily a result of not receiving an oral oncolytic drug. Additional details on the reasons for study exclusion are shown in Figure 2.
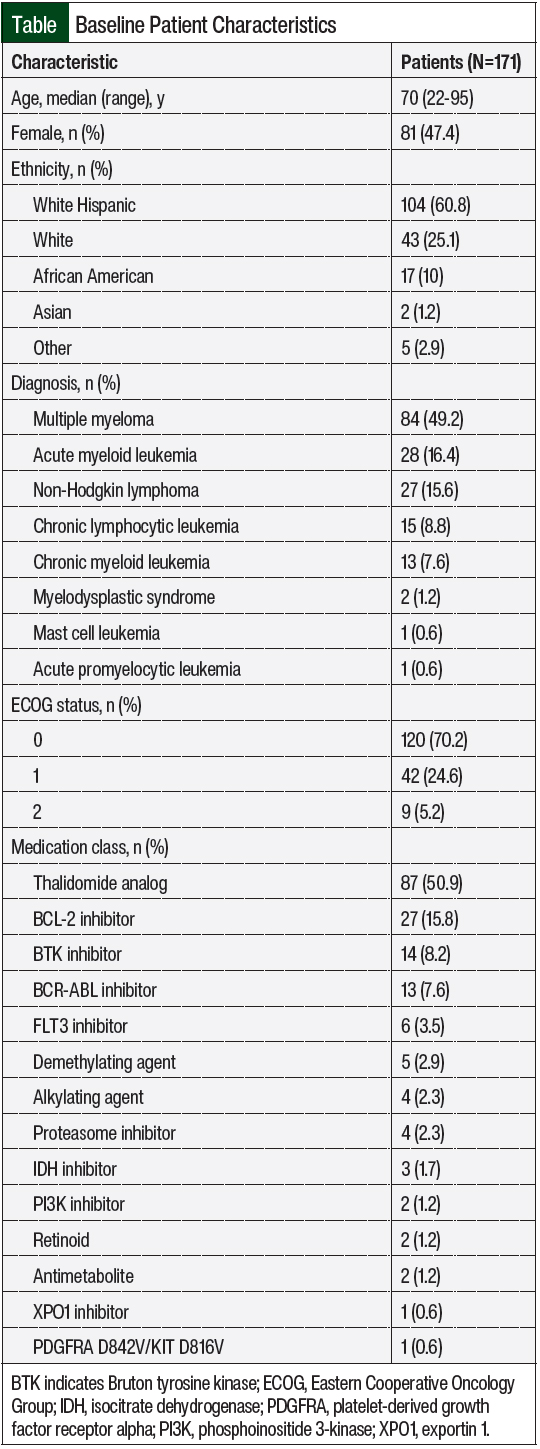
The patients who were included in the review were predominantly White Hispanic (60.8%), with nearly half (49.2%) of the patients diagnosed with multiple myeloma. Most (70.2%) of the patients had an Eastern Cooperative Oncology Group status score of 0, and the most prescribed (50.9%) medications were thalidomide analogs (Table).
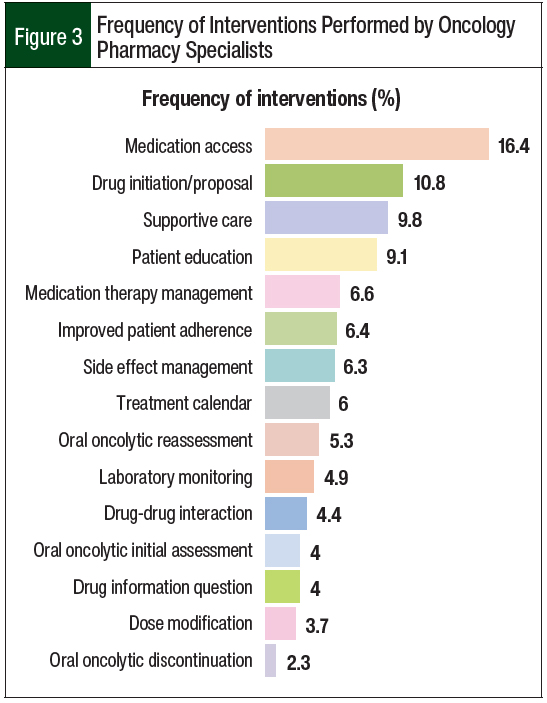
The most common interventions performed by oncology pharmacy specialists were addressing medication access (16.4%), followed by drug initiation/proposal (10.8%), supportive care management (9.8%), patient education (9.1%), and medication therapy management (6.6%; Figure 3).
The most common interventions in the supportive care category were immunizations (41%), the management of electrolytes (11%), infection prophylaxis (9%), the management of bone-modifying agents (7%), and the management of nausea or vomiting (6%). Other supportive care interventions included the management of skin reactions, gastrointestinal side effects, mucositis, pain, anxiety, and tumor lysis prophylaxis.
Discussion
This study evaluated the role of oncology pharmacy specialists in oral oncolytics management in the hematology clinic and, in doing so, identified specific challenges in the management of oral oncolytic drugs.
In a 6-month period, oncology pharmacy specialists performed 1213 interventions, yet only 701 interventions related to the management of oral oncolytic drugs for 171 patients were included in this study. A majority of interventions were excluded from the study because they were related to the management of intravenous chemotherapy, the management of internal medicine issues unrelated to oral oncolytic drugs, patients having a benign disease, or patients being enrolled in a clinical trial. The pharmacy specialists most often intervened on medication access (16.4%), drug initiation/proposal (10.8%), supportive care (9.8%), patient education (9.1%), and medication therapy management (6.6%).
Insurance authorization is one of the major reasons for a delay in receiving oral oncolytic therapy in previous studies.17,18 In this review, medication access was a significant challenge, accounting for the majority of interventions performed by oncology pharmacy specialists. Medication access interventions consisted of measures taken by the pharmacy specialist to ensure that therapy was received in a timely manner, such as help with insurance authorizations, clinical justification in the form of an appeal letter or peer-to-peer call, and prescription transfers to the insurance provider’s in-network or contracted specialty pharmacy.
A frequent intervention was drug initiation/proposal, which consisted of proposing an oral oncolytic treatment plan based on the most up-to-date literature while taking into consideration patient-specific factors such as comorbidities, renal and hepatic functions, and drug-drug interactions. This intervention required specialized pharmacotherapy training, an integrated provider team collaboration, and patient involvement in joint decision-making. This particular role of oncology pharmacy specialists is described in several studies.19-21 From a legislative perspective, 43 states now allow collaborative practice agreements where oncology pharmacists engage patients as mid-level practitioners with expanded privileges such as oral oncolytic prescribing, ordering dose adjustments, processing refills, and discontinuing medications.22,23 According to the American Society of Clinical Oncology’s projection of a growing need for oncology care, oncology pharmacists, nurse practitioners, and physician assistants will be increasingly needed.24,25 An estimated 2.9 million to 4.1 million 30-minute patient visits led by oncology pharmacists are expected to reduce provider workload by 50% from 2020 to 2025.25 This review demonstrates the contributions that oncology pharmacy specialists can have on oral oncolytic therapy management, specifically as it relates to therapy optimization, supportive care management, and medication access.
Most of the supportive care interventions in this review were related to immunizations (41%). The most common vaccines proposed by oncology pharmacy specialists were pneumococcal (Prevnar 13 and Pneumovax 23); hepatitis A and B (Twinrix); diphtheria, tetanus, and pertussis (DTaP); haemophilus influenzae type B (Hib); meningitis (Menveo); polio (IPV); measles, mumps, and rubella (MMR); and chickenpox (Varicella and Shingrix). Immunization interventions were made primarily for patients who were receiving oral oncolytic therapy and underwent autologous or allogeneic stem cell transplant.
The second most common intervention in the supportive care category was the management of electrolytes (11%). Some oral oncolytics, such as venetoclax, thalidomide analogs, and tyrosine kinase inhibitors, are associated with a higher rate of electrolyte abnormalities, particularly as a result of poor nutrition, emesis, diarrhea, or tumor lysis syndrome.26 The timely recognition and mitigation of electrolyte loss, especially if oral oncolytics are combined with chemotherapy, may prevent life-threatening conditions.
The third most common intervention in the supportive care category in this study was infection prophylaxis (9%). Infection is a frequent adverse event of chemotherapy, which requires risk stratification for each patient.27 The most frequent infection prophylaxis interventions identified in this study were related to drugs prescribed for viral and fungal prophylaxes.
One of the most frequently identified interventions in this study was patient education (9.1%), which involved interacting with a patient in person or by phone to discuss the appropriate use of oral oncolytics, including their indication, dose, frequency, Risk Evaluation and Mitigation Strategy (REMS) requirements, common side effects, and management of adverse events, and what to expect during a follow-up visit. One of the ways to mitigate nonadherence is to provide patient education by oncology pharmacy specialists. This study further establishes the oncology pharmacy specialist’s role in reinforcing patient adherence, which was the sixth most common intervention performed in the outpatient hematology clinic.
The medications that were most frequently intervened on included lenalidomide, pomalidomide, and venetoclax, which align with the most frequent disease states of multiple myeloma and acute myeloid leukemia at MCI. Thalidomide analogs (lenalidomide, pomalidomide) for the treatment of multiple myeloma accounted for 50.9% of all interventions. This is consistent with what is known about multiple myeloma as a disease state because thalidomide analogs are key components of treatment regimens for newly diagnosed and relapsed multiple myeloma. Therapy for multiple myeloma includes complex multidrug regimens with special dosing schedules associated with distinct supportive care recommendations (venous thromboembolism prophylaxis), special medication access requirements (REMS program), and complicated side-effect profiles and monitoring parameters (thyroid monitoring, pregnancy testing, renal adjustments) that oncology pharmacy specialists in the outpatient clinic have the skill set and training to help manage.
Limitations
This study is limited by its single-arm, descriptive nature, as well as by its retrospective study design that is restricted to the subjectivity that comes with documentation. Intervention data were also manually categorized based on documentation provided by oncology pharmacy specialists in patient charts, which relied on the reviewer’s interpretation of each intervention.
Conclusion
This review demonstrates the important clinical role of oncology pharmacy specialists who manage oral oncolytics in the outpatient hematology setting, particularly in the treatment and monitoring of patients with multiple myeloma who are receiving thalidomide analogs, such as lenalidomide and pomalidomide. By providing expert medication recommendations, expediting medication access, and managing adverse events, oncology pharmacy specialists are well-positioned to contribute to medication access, therapy optimization, and the supportive care management of patients with hematologic malignancies who are receiving oral oncolytic therapy.
Oncology pharmacy specialists should continue to expand support in the outpatient setting to optimize the management of patients with hematologic malignancies who are receiving oral oncolytics. Future prospective studies are needed to fully describe the impact of oncology pharmacy specialist–driven interventions on the management of oral oncolytics as it relates to time and cost-savings, using additional end points such as financial outcomes of each intervention on the institution and individual patients. Such future studies may further prove the necessity for hiring additional pharmacists in this setting.
Author Disclosure Statement
Dr Davenport, Dr Tadros, Dr Al Sagheer, Dr Roy, Dr Rubens, Dr Ramos Morales, and Dr Shwin have no conflicts of interest to report.
References
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758-2765.
- Colby SL, Ortman JM; for the US Census Bureau. The baby boom cohort in the United States: 2012 to 2060. May 2014. www.census.gov/library/publications/2014/demo/p25-1141.html. Accessed June 2, 2022.
- Ward S, Olson N. Approval of oral oncolytics: 1995-2020. www.ncoda.org/wp-content/uploads/2021/10/Approval-of-Oral-Oncolytics-1995-2020-Ward.pdf. Accessed June 2, 2022.
- Birner A. Pharmacology of oral chemotherapy agents. Clin J Oncol Nurs. 2003;7(6 suppl):11-19.
- Stuurman FE, Nuijen B, Beijnen JH, Schellens JHM. Oral anticancer drugs: mechanisms of low bioavailability and strategies for improvement. Clin Pharmacokinet. 2013;52:399-414.
- Desai A, Gyawali B. Financial toxicity of cancer treatment: moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine. 2020;20:100269.
- Kleinsinger F. The unmet challenge of medication nonadherence. Perm J. 2018;22:18-033.
- Nachar VR, Farris K, Beekman K, et al. Clinician report of oral oncolytic symptoms and adherence obtained via a patient-reported outcome measure (PROM). JCO Clin Cancer Inform. 2019;3:1-6.
- Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. 2016;21:354-376.
- Jacobs JM, Ream ME, Pensak N, et al. Patient experiences with oral chemotherapy: adherence, symptoms, and quality of life. J Natl Compr Canc Netw. 2019;17:221-228.
- Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86:471-474.
- O’Brien S, Berman E, Moore JO, et al. NCCN Task Force report: tyrosine kinase inhibitor therapy selection in the management of patients with chronic myelogenous leukemia. J Natl Compr Canc Netw. 2011;9(suppl 2):S1-S25.
- Jiao Q, Bi L, Ren Y, et al. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer. 2018;17(1):36.
- Darling JO, Raheem F, Carter KC, et al. Evaluation of a pharmacist led oral chemotherapy clinic: a pilot program in the gastrointestinal oncology clinic at an academic medical center. Pharmacy (Basel). 2020;8(1):46.
- Levitan D. Oncology pharmacists can improve TKI adherence in CML. Cancer Network. November 11, 2020. www.cancernetwork.com/view/oncology-pharmacists-can-improve-tki-adherence-cml. Accessed June 2, 2022.
- Battis B, Clifford L, Huq M, et al. The impacts of a pharmacist-managed outpatient clinic and chemotherapy-directed electronic order sets for monitoring oral chemotherapy. J Oncol Pharm Pract. 2016;23:582-590.
- Wang AA, Tapia C, Bhanji Y, et al. Barriers to receipt of novel oral oncolytics: a single-institution quality improvement investigation. J Oncol Pharm Pract. 2020;26:279-285.
- Raborn ML, Pelletier EM, Smith DB, Reyes CM. Patient out-of-pocket payments for oral oncolytics: results from a 2009 US claims data analysis. J Oncol Pract. 2012;8(3 suppl):9S-15S.
- Biltaji E, Yoo M, Jennings BT, et al. Outcomes associated with pharmacist-led diabetes collaborative drug therapy management in a Medicaid population. J Pharm Health Serv Res. 2017;8:59-62.
- Waliji-Banglawala A, Patel S, Hurley SL, Condon P. What can your pharmacist do for you? Role of collaborative drug therapy management in hospice (QI641). J Pain Symptom Manage. 2020;59:521-522.
- Tewksbury A, Bozymski KM, Ruekert L, et al. Development of collaborative drug therapy management and clinical pharmacy services in an outpatient psychiatric clinic. J Pharm Pract. 2018;31:272-278.
- Mackler E, Segal EM, Muluneh B, et al. 2018 Hematology/Oncology Pharmacist Association Best Practices for the Management of Oral Oncolytic Therapy: Pharmacy Practice Standard. J Oncol Pract. 2019;15(4):e346-e355.
- Merten JA, Shapiro JF, Gulbis AM, et al. Utilization of collaborative practice agreements between physicians and pharmacists as a mechanism to increase capacity to care for hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2013;19:509-518.
- Yang W, Williams JH, Hogan PF, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract. 2014;10:39-45.
- Knapp K, Ignoffo R. Oncology pharmacists can reduce the projected shortfall in cancer patient visits: projections for years 2020 to 2025. Pharmacy (Basel). 2020;8(1):43.
- Verzicco I, Regolisti G, Quaini F, et al. Electrolyte disorders induced by antineoplastic drugs. Front Oncol. 2020;10:779.
- Nucci M, Anaissie EJ. Prevention of infections in patients with hematological malignancies. In: Wiernik PH, Dutcher JP, Gertz MA, eds. Neoplastic Diseases of the Blood. 6th ed. Springer International Publishing AG; 2018:1047-1062.