Understanding adverse drug reactions (ADRs) and the application of pharmacovigilance are necessary to provide safe and effective patient care. Institutional policies at Sibley Memorial Hospital (SMH)-Johns Hopkins Medicine in Washington, DC, define ADRs as any undesirable reaction (symptom or syndrome) that occurs when a patient receives a drug for diagnostic, prophylactic, or therapeutic indications. The World Health Organization defines pharmacovigilance as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other medicine/vaccine related problem.”1 The current literature describes various methods by which pharmacists can achieve pharmacovigilance in oncology clinical practice.2 Finn and colleagues completed a retrospective chart review that compared pharmacists’ interventions before and after the implementation of the EPIC Beacon medical oncology module electronic health record (EHR), with a goal of evaluating and quantifying the number of interventions made by clinical pharmacists.2 This study showed a 6-fold increase in pharmacovigilance observations via pharmacist interventions with the implementation of Beacon in the EHR.2 Although the findings of this study show the beneficial impact of pharmacists, this method has not been transferrable to current practices for ADR monitoring at SMH, despite our established use of the same technology.
When examining the process of capturing ADRs, the literature provides methods to aid the identification of these reports, as well as to create clinically impactful interventions.3-5 Brockstein and colleagues discussed the use of treatment notes with the addition of built-in laboratory test standardization in the EHR as a means of identifying ADRs and improving medication outcomes for patients.3 Examples of ADR prevention and detection standardization implemented in this study included requiring a urine protein creatinine ratio and blood pressure order for patients receiving bevacizumab after 31% of patients had bevacizumab-induced hypertension. The outcomes of this study were to improve the safe administration of high-risk medications, as well as to trend medication errors. The study results showed that over a 6-month period, the treatment note allowed clinical pharmacist reviewers to identify and prevent a total of 5 potential dose-modification omissions.3
In addition, in the Calling for Early Detection of Adverse Reactions (CEDAR) project, Schiff and colleagues advanced real-time ADR surveillance, the expertise of pharmacists, and the documentation of ADRs in the EHR within the clinical setting for patients with 4 common chronic conditions.4 Their proactive methodology used an EHR-integrated interactive voice response combined with live phone transfer to a pharmacist (when ADRs were reported by patients) to detect and evaluate ADRs beyond the scope achieved with conventional methods performed in the practice.4
In a recent literature review designed to identify the current challenges in the detection of medication-related symptoms and assessment of technology-based opportunities to increase pharmacovigilance, Salazar and colleagues from 3 academic medical centers described a stepwise approach, best practices, and lessons learned from the CEDAR project.5 In this proactive ADR detection and evaluation system, the role of the pharmacist is essential.5
The findings of Brockstein and colleagues, Schiff and colleagues, and Salazar and colleagues lay a foundation within their respective institutions for the implementation of an alternative method for capturing suspected ADR data, increasing reporting capabilities, and assessing patient outcomes.3-5 Based on previous publications, it can be implicated that leveraging clinical pharmacists and technology to find more effective and efficient ways to capture and assess ADR data improves medication safety while concurrently identifying areas for improvement.3-5
It is well documented in the healthcare sector that patients who are receiving antineoplastic or oncolytic agents have a high incidence of ADRs6,7; however, the use of the EHR to capture infusion center–related ADRs continues to be an area of interest for clinical pharmacists, as well as the entire oncology care team.6 Because oncolytic agents have a burdensome ADR profile, adverse events resulting from these medications are oftentimes underreported within clinical trials and clinical quality improvement activities.7 An unpublished annual report conducted at SMH in 2020 revealed that more than 40% of the total reported adverse events were associated with antineoplastic agents. SMH has a policy that states that any change in chemotherapy dose or agent must be documented in the modification section of the treatment note in the EHR. Whether these modifications result from suspected ADRs is a quality improvement area that our research sought to assess.
There are several methods of reporting suspected ADRs at SMH. SMH has a policy that healthcare professionals must report all suspected ADRs. The first standard method is reporting by healthcare professionals through the proper institutional channels, including an internal events reporting system and the use of an ADR form by clinicians. There is also an ADR hotline and ongoing review by clinical pharmacists of ADR-related International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes available as standard methods of reporting. The ADR hotline and clinicians’ use of ADR forms have the lowest use for reporting. The internal event reporting system and ADR detection by clinical pharmacists via review of ICD-10-CM codes have the highest use for reporting within the infusion center at SMH.
Mandated healthcare professional reporting, reviewing ICD-10-CM codes, and calling in via the ADR hotline are recognized to not fully capture the suspected ADRs documented by the prescriber in the EPIC Beacon treatment note. This study aims to address this gap by testing an alternative method to capture and analyze suspected ADR data among patients who received treatment at the SMH infusion center. The primary objective of this study is to quantify the number of suspected ADRs identified via the alternative method versus standard methods. The second objective is to identify patterns and types of suspected ADRs in the alternative method versus standard methods. The third objective is to identify opportunities for improvement in the use of chemotherapy in patient care.
Methods
This single-center, retrospective, cross-sectional study evaluated data from January 1, 2020, to December 31, 2020. The data collection was automated for the first time in the SMH infusion center via EHR reporting tools for patients who were receiving chemotherapy or biologic agents with treatment adjustments (treatment note modifications). Next, a sample of entries were reviewed by the research team that was composed of 2 clinical pharmacists, 1 infusion center pharmacy manager, and 1 pharmacy practice resident to identify suspected ADRs solely from the generated EPIC report. The records were categorized as ADR or non-ADR.
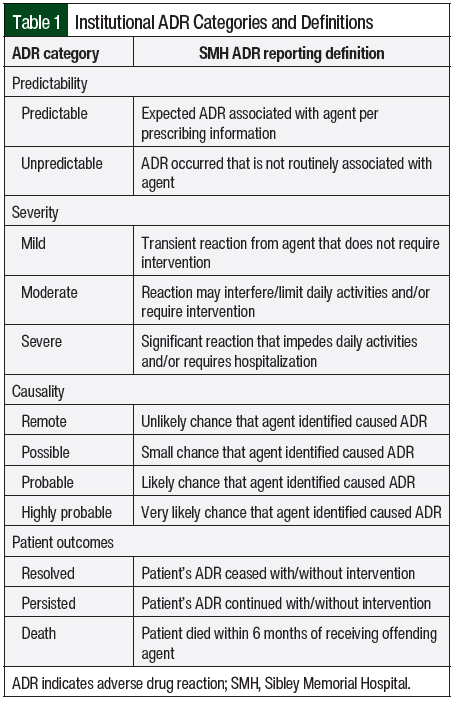
We assessed a representative sample of eligible patients for the extrapolation of annualized ADRs. This targeted approach sought to reach 20 confirmed ADR records. A total of 20 entries were selected because of the amount of time needed for manual chart review to identify that an ADR occurred and subsequent completion of the SMH ADR antineoplastic form (Appendix). Per SMH’s institutional policies, these patient-level suspected ADRs were classified by predictability, severity, causality, and patient outcomes (Table 1). The patient outcomes were classified as symptom resolution, symptom persistence, or patient death. The patient notes were reviewed up to 6 months after the ADR was initially associated with the treatment note modification. The Naranjo algorithm was used to aid in the determination of the probability of an ADR (ie, causality, which is defined in this study as the likeliness of a particular drug causing an ADR).8
The study inclusion criteria were age ≥18 years and being seen at the infusion center at SMH, having an order for chemotherapy and/or a biologic agent, and having a treatment modification in the chemotherapy treatment note between January 2020 and December 2020. The study’s exclusion criteria were receiving treatment somewhere other than at SMH and not having treatment modifications made by SMH’s oncologists. Descriptive analysis was used to dissect the confirmed ADRs. The process for validating the data included manually reviewing the chart of each patient in the sample to confirm that the modifications met the inclusion criteria and that further healthcare professional notation within the EHR (ie, progress note documentation, nursing note) provided a description of the ADR that occurred.
Once the ADRs were categorized, the opportunities for improvement were identified via a stepwise approach. The first step was to review all antineoplastic ADRs captured via standard methods in SMH’s 2020 ADR Annual Report to assess the extent of missed or duplicate ADR reporting compared with the new approach via the EHR. The second step was to provide actionable recommendations to increase pharmacovigilance based on the research findings.
Results
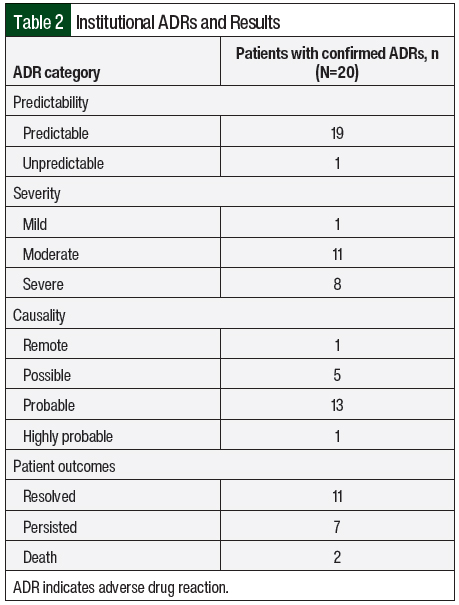
A total of 331 treatment modification entries were collected from the EHR. Of these entries, 88 (26.6%) were reviewed to attain 20 confirmed and fully assessed ADRs. A total of 68 (77.3%) of the entries reviewed were categorized as non-ADRs, whereas 20 (22.7%) were categorized as confirmed ADRs (Table 2). For predictability, 19 (95%) of the confirmed ADRs were predictable in nature (ie, not classified as a hypersensitivity or idiosyncratic reaction), whereas 1 (5%) was unpredictable (ie, a hypersensitivity or idiosyncratic reaction). For severity, 1 (5%) ADR was mild, 11 (55%) were moderate, and 8 (40%) were severe. For causality, 1 (5%) of the confirmed ADRs was remote, 5 (25%) were possible, 13 (65%) were probable, and 1 (5%) was highly probable. For patient outcomes, 11 (55%) patients had resolved ADRs, 7 (35%) had persistent ADRs, and 2 (10%) patients died.
Of the 2 deaths that occurred, 1 resulted from ADRs associated with oxaliplatin treatment and 1 with afatinib treatment. In the oxaliplatin-related death, the patient initially received a dose of 85 mg/m2 for the salvage treatment of pancreatic cancer and had neuropathy. Neuropathy is a common (and sometimes irreversible) occurrence associated with oxaliplatin treatment that typically requires a dose reduction and/or dose discontinuation.9 For the management of the ADR, the dose of oxaliplatin was reduced to 65 mg/m2. Unfortunately, the cancer progressed rapidly, after which chemotherapy was stopped, and the patient transitioned to hospice and died shortly thereafter.
In the afatinib-related death, the patient initially received afatinib 30 mg for the treatment of lung cancer and had severe nausea. Although treatment with afatinib, an endothelial growth factor receptor inhibitor, has resulted in nausea, diarrhea is a more frequently associated ADR with afatinib.10 For the management of the ADR, the afatinib dose was reduced to 20 mg and the patient was prescribed 2 antiemetic medications to take at home. The oncologist recommended holding chemotherapy, but after the patient expressed concern about the cancer progressing, the provider decided to continue treatment with afatinib. The patient died within 3 months of treatment initiation with afatinib. Oxaliplatin and afatinib were dosed according to their respective prescribing information.
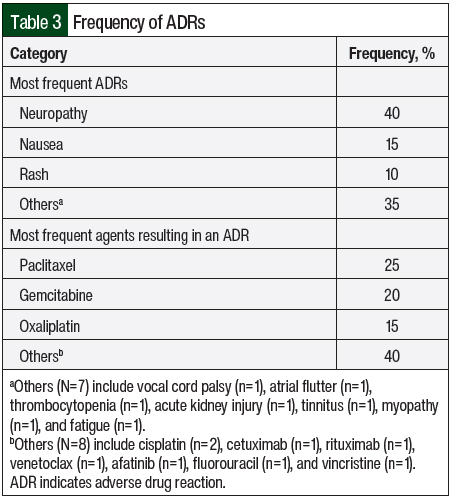
Neuropathy was the most frequent ADR identified in the total cohort (40%), followed by nausea (15%), rash (10%), and “other reactions” (35%; Table 3). Paclitaxel was the most frequent agent resulting in an ADR (25%), followed by gemcitabine (20%), oxaliplatin (15%), and “other agents” (40%). In terms of frequent relationships between ADRs and antineoplastic agents, paclitaxel was the most often associated with neuropathy. Other agents did not have frequent associations with the ADRs listed. None of the 20 ADRs that were identified were previously reported in the SMH 2020 ADR Annual Report. In other words, there was no overlap or duplication of ADR reporting. Comparing the 2 methods, the alternative method was able to capture ADRs that the institution’s conventional methods of reporting did not capture.
Discussion
Of the reviewed treatment note modifications entries, 25% were made by the oncology provider as a result of a suspected ADR. The review of 88 entries was required to reach 20 confirmed ADRs. Based on the study findings and the 331 total chemotherapy note modifications identified in 2020, it can be extrapolated that 75 previously unreported ADRs (approximately 25%) occurred in 2020 within the SMH infusion center, had all 331 modifications been reviewed by the research team. The patients identified in this study had a significant event from their chemotherapy regimens such that their treatment(s) required a dose reduction, delay, and/or discontinuation. These findings highlight the need for advancing current ADR reporting methods aided by EHR-facilitated quality improvement activities of the clinical pharmacy services to advance pharmacovigilance.
The existing infusion center pharmacy staffing plan at SMH includes, aside from pharmacists and certified pharmacy technicians who provide pharmaceutical supplies and services, 1 nondispensing clinical pharmacist daily who is assigned to prospectively review treatment plans and 1 nondispensing clinical pharmacy manager. The new method for pharmacovigilance described in this report was feasible because of the assistance of a pharmacy practice resident and limited support from the inpatient clinical pharmacy manager who oversees the SMH ADR program. Strategies to sustain this new approach at our institution and/or to initiate and perform this work at similarly staffed oncology infusion centers requires the reassignment of existing lower-priority work responsibilities, the use of advanced pharmacy learners, and/or a supplementary pharmacy budget for the addition of qualified personnel. Further research on the approach used at our infusion center should provide insight into the feasibility and generalizability of our findings to other high-volume oncology infusion center practices.
There are several opportunities for improvement that have been identified through this research. These opportunities include reducing the time to fill out the SMH ADR antineoplastic form, improving clinical documentation in the treatment note modification, consistently documenting oncology drug allergies and intolerances in the EHR, and escalating ADR-related dose modifications to the proper institutional channels for reporting. The researchers who filled out the ADR form timed themselves from beginning to end and recorded the completion time in a spreadsheet. The average time for the completion of an ADR was 42 minutes. Recommendations to reduce the time to complete the form while providing a complete summary of the ADR include having online and paper versions, as well as having an open section for pertinent laboratory values rather than listing every laboratory value that is available.
In regard to improving clinical documentation, currently the rationale section of the treatment note is a free-text field that oncology providers are not required to fill out. The recommendations to improve this area include using keyword indicators described in the results section (eg, reduce, omit) to determine whether a suspected ADR has occurred. Using keyword indicators would allow for a more efficient capture of suspected ADRs.
For documenting allergies or intolerances, all 20 patients who had significant ADRs that resulted in a change to or a discontinuation of therapy did not have their reaction documented in the appropriate section of the EHR. The recommendations to improve this documentation include educating healthcare personnel (eg, nurses, oncologists, clinical pharmacists) on the use of documenting allergies and significant intolerances in the appropriate location. The use of the “allergy/intolerance” section of the EHR for ADR documentation would send an alert for the drug that resulted in the ADR if the providers attempt to inadvertently or inappropriately place or reinstitute full-dose chemotherapy orders for the patient.
Last, for institutional quality improvement reporting of ADRs via treatment note modifications, none of the 20 confirmed ADRs were reported via the standard methods. The researchers suspect that this is a result of the low level of reporting by infusion center staff and oncology providers combined with the high level of expected or predicted ADRs (95%) associated with those that were identified. For example, because oxaliplatin has a predictable adverse-event profile of resulting in neuropathy, providers may be less likely to further escalate the ADR to the proper channels and will simply choose to omit or reduce the dose in these settings. The alternative method implemented in this study has not been well described in literature and contributes to closing the ADR reporting gaps. In addition, this method offers an automated yet highly clinical process to capture and analyze ADRs, which leads to improved pharmacovigilance. It is essential to leverage the expertise of the clinical pharmacists within the organization to efficiently capture and analyze ADRs in the infusion center’s patient population at SMH. It is important to acknowledge that some institutions may not prioritize this quality improvement activity or deploy the resources needed to support taking on clinical pharmacists in this important role.
The purpose of this study was to find a more effective and efficient means to capture ADRs in the ambulatory oncology infusion center population. These findings show that a more automated and integrated means for pharmacovigilance is a worthwhile pursuit, especially in the ambulatory oncology infusion therapy population. While being mindful of the site-specific processes that already exist at SMH, our research can be used as a blueprint for the implementation of a more proactive method to capture and analyze important chemotherapy-related ADRs.
Limitations
This study has several limitations. The first and most important limitation identified from pharmacovigilance and quality improvement perspectives was that any treatment plans that were discontinued were not part of the data set. As a result, a cluster of potential ADRs that were severe enough that the treatment had to be discontinued were not included. In subsequent research efforts, this gap can be addressed by adding in a parameter for the inclusion of discontinued treatments, should the automated report functionality allow for it.
The next limitation identified was a process-related issue, in that the data collected did not include the dates of the treatment modifications. As a result, the researchers spent additional time reviewing the EHR to validate that the entry was within the targeted 2020 time frame. In subsequent research efforts, this gap can be addressed by manipulating the report to display modification dates in a human-readable format (eg, MM/DD/YY) rather than a machine-readable format (eg, 65799, 65743).
A methodologic limitation was the extrapolation of the ADR cases from a representative sampling of the 331 chemotherapy modification entries. Because each antineoplastic ADR form averaged 42 minutes to complete, the impact of time constraints in the pharmacy residency year limited the research to a feasible goal of 20 confirmed ADRs. This, coupled with the additional time to manually review the patients’ charts, compelled the researchers to tailor the number of entries that were necessary to reach a goal of 20 ADRs, which, in this case, was 88 treatment note entries.
The final limitation that was identified was that the exclusion criteria did not encompass oral oncology drugs. As a result, 2 of the 20 ADRs identified concomitant infusion therapy and oral chemotherapy. These patients were captured and were ultimately included in the data analysis because they fit the inclusion criteria as a result of receiving an infusion regimen at some point in 2020. Because oral agents are not administered in the infusion center at SMH, treatment note modifications that result from the use of these agents do not reflect the ADRs that the researchers initially intended to capture.
Conclusion
The alternative method used in this study to enhance pharmacovigilance in the oncology infusion center has been proven to capture ADRs that are not currently identified via standard quality improvement methods of reporting at SMH. Further analysis of the confirmed ADRs to identify educational and prevention measures should prove beneficial in improving the care of the patients, as well as in advancing the role of the clinical pharmacists.
The identified opportunities for improvement (including decreasing ADR form fill-out time, increasing provider modification documentation, increasing provider allergy or tolerance documentation, and escalating ADR-related modifications) will allow for this alternative method to improve pharmacovigilance for patients who receive care at the SMH infusion center. Similar to experiences at SMH, it is anticipated that an increasing number of oncology infusion center practices will adopt proactive, technology-aided pharmacovigilance approaches to improve patient care.
Acknowledgements
The researchers would like to acknowledge the Department of Pharmacy and the Department of Oncology at SMH for their assistance and support with the implementation of this study.
Author Disclosure Statement
Dr Leonard, Dr Goldenhorn, Dr Keeys, and Dr Ali have no conflicts of interest to report.
References
- World Health Organization. What is pharmacovigilance? www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance#:~:text=Pharmacovigilance%20is%20the%20science%20and,they%20are%20authorized%20for%20use. Accessed September 11, 2023.
- Finn A, Bondarenka C, Edwards K, et al. Evaluation of electronic health record implementation on pharmacist interventions related to oral chemotherapy management. J Oncol Pharm Pract. 2017;23:563-574.
- Brockstein B, Hensing T, Carro GW, et al. Effect of an electronic health record on the culture of an outpatient medical oncology practice in a four-hospital integrated health care system: 5-year experience. J Oncol Pract. 2011;7e20-e24.
- Schiff GD, Klinger E, Salazar A, et al. Screening for adverse drug events: a randomized trial of automated calls coupled with phone-based pharmacists counseling. J Gen Intern Med. 2019;34:285-292.
- Salazar A, Amato MG, Shah SN, et al. Pharmacists’ role in detection and evaluation of adverse drug reactions: Developing proactive systems for pharmacosurveillance. Am J Health Syst Pharm. 2023;80:207-214.
- Kollu V, Letendre P, Oettel KR, et al. Outcomes of a quality improvement project integrating an EHR (electronic health record) for improved adverse drug reaction (ADR) communication at an adult outpatient cancer infusion center. J Clin Oncol. 2022;40:372.
- Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. Am J Clin Oncol. 2015;33:910-915.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Hertz D, Dockter TJ, Satele DV, et al. Neuropathy severity at the time of oxaliplatin treatment alteration in patients with colon cancer (Alliance A151912). Support Care Cancer. 2021;29:7855-7863.
- Yokota H, Sato K, Sakamoto S, et al. Relationship between plasma concentrations of afatinib and the onset of diarrhea in patients with non-small cell lung cancer. Biology (Basel). 2021;10:1054.