In 2023, nearly 20,000 women will be diagnosed with ovarian cancer in the United States and more than 13,000 will die from this disease.1 Ovarian cancer is one of the 5 leading causes of cancer death in American women and carries a 5-year overall survival rate of 50.8%.1 Defects in BRCA1 and BRCA2 increase the risk for epithelial ovarian cancer.2,3 Approximately 20% of cases of epithelial ovarian cancer are associated with inherited (germline) BRCA1/2 mutations, whereas up to 9% are associated with somatic/tumor BRCA1/2 mutations.4-6 The risk for ovarian cancer by age 80 years increases by 44% and 17% for patients with BRCA1 and BRCA2 mutations, respectively.3 Currently, the American Society of Clinical Oncology recommends germline mutation testing (including BRCA1/2) for all women with ovarian cancer, and somatic tumor testing for anyone who does not carry a germline mutation.7
BRCA1 and BRCA2 are tumor suppressor genes that are involved in the process of homologous recombination repair (HRR) of double-stranded DNA breaks.8 When these genes mutate, cellular repair machinery is dysfunctional. Further characterization of the genetic landscape of ovarian cancer has demonstrated high rates of loss of heterozygosity (LOH) in cancers with germline BRCA mutation.8 In contrast, a molecular analysis of a pan-cancer population showed that LOH in patients with somatic BRCA mutation–positive cancers is rare.9 These findings led to the hypothesis that somatic BRCA1/2 mutations, when not accompanied by other markers of homologous recombination, may not drive tumorigenesis to the same degree as germline events and/or LOH status, and thus may lead to different responses to therapy.9
LOH, accompanied by a germline BRCA mutation loss of function mutation, results in complete functional loss of the BRCA protein, and thus more severe predicted deficiencies in HRR when compared with patients who have a single allele loss of function variant (ie, they have 1 functional BRCA allele intact).9,10 Studies suggest that LOH may be another marker to predict response to poly (ADP-ribose) polymerase (PARP) inhibitors in ovarian cancer, either alone or in combination with BRCA1/2 mutation status.9,10 Other markers of homologous recombination deficiency (HRD), including telomeric allelic imbalance, large-scale state transitions, and genomic instability scores, may also be useful in characterizing tumors who have an HRD phenotype.10,11 There are several assays that have been evaluated to identify the level of HRD in cancer tissue, but no one test is currently recommended in guidelines for the treatment of ovarian cancer, despite certain PARP inhibitors with companion diagnostics that include HRD status.10,11
Deficiencies in the HRR pathway support the use of agents that introduce or exploit double-stranded DNA breaks, which then cannot be repaired by HRR, including cytotoxic chemotherapy and PARP inhibitors.12 Platinum-based chemotherapy is the standard therapeutic strategy for the treatment of ovarian cancer.11 Ovarian tumors with defects in BRCA1/2 have higher response rates to platinum-based chemotherapy, possibly because of a lesser ability to correct DNA damage resulting from treatment with platinum agents.14 Patients who have platinum-sensitive, recurrent ovarian cancer (which is defined as a relapse >6 months after the most recent platinum-based chemotherapy), will frequently be re-treated with further platinum-based agents.11 Over time, as a patient is exposed to further platinum chemotherapy and their disease progresses, the likelihood of response and the duration of benefit observed decrease.11 Approximately 70% of patients with ovarian cancer will have a relapse after the initial treatment.13,15 The probability of response and prognosis of relapsed patients is related to disease progression and relapse-free interval from the last platinum therapy (platinum-free interval; PFI). Patients with platinum-refractory disease (PFI within 4 weeks) and platinum-resistant disease (PFI within 6 months) typically have a low response rate and poor prognosis. On the other hand, patients who are platinum partially sensitive (PFI of 6-12 months) and platinum sensitive (PFI >12 months) have better prognosis and longer survival.13,15 The treatments used in the platinum-refractory setting have limited efficacy.11
Given the limited therapeutic options available for patients with platinum-refractory or platinum-resistant ovarian cancer, targeted therapies, including PARP inhibitors, emerged as an exciting treatment option. PARP constitutes a family of enzymes that are involved in the repair of DNA nicks, double-stranded breaks, and homologous recombination.15,16 The inhibition of PARP enzymes in the setting of additional defects in homologous recombination (such as BRCA1/2 loss of function) leads to genomic instability and sometimes cell death. The mechanism of PARP inhibitors to induce cell death in the setting of HRD is known as synthetic lethality.15,16
Olaparib is a first-in-class PARP inhibitor that was approved by the FDA in 2014 for the treatment of advanced ovarian cancer in patients with germline BRCA mutations who have received ≥3 lines of chemotherapy.17 Since olaparib’s accelerated approval, multiple trials have shown the success of PARP inhibitors as treatment and maintenance therapy for ovarian cancer, leading to the approval of 2 other agents, rucaparib and niraparib.18-20
A review of randomized controlled trials conducted with platinum-based chemotherapy and PARP inhibitor therapy revealed that platinum sensitivity is a predictive biomarker for PARP inhibitor response.13 Specifically, BRCA1/2-positive ovarian cancers exhibit high sensitivity to platinum and PARP inhibitors, and the overall survival rate is higher in these patients than in patients who do not have BRCA1/2-positive ovarian cancer.13 Most studies involving PARP inhibitors focus on patients with germline BRCA mutation given their increased prevalence, although smaller studies have shown promising efficacy in patients with somatic tumor mutations.4,21 In a meta-analysis conducted by Mohyuddin and colleagues, 5 studies showed similar progression-free survival (PFS) rates in patients with germline and somatic BRCA-positive ovarian cancer who received PARP inhibitors, but the researchers recommended further investigation of the use of PARP inhibitors in patients with somatic BRCA-positive ovarian cancers to fully assess efficacy in this population.22 Although the National Comprehensive Cancer Network guidelines recommend treatment with PARP inhibitors in the maintenance setting in patients with germline and somatic BRCA mutations,11 further characterization of the differences in benefit in these distinct populations is warranted to improve patient care.
We sought to characterize the duration of maintenance therapy with PARP inhibitors in patients with ovarian cancer at our institution based on germline or somatic BRCA mutation status. Our additional investigative aims included to assess the rates of treatment-related adverse events and therapy changes, as well as to characterize our use of LOH and HRD testing and BRCA mutation analysis.
Methods
This single-center, retrospective, observational study was approved by the research ethics institutional review board. The initial data pull was from electronic health records (EHRs) and was based on the study’s inclusion criteria. Patients were included in the study if they were diagnosed with primary epithelial ovarian, fallopian tube, or peritoneal cancer and received outpatient maintenance therapy with PARP inhibitors at the University of North Carolina (UNC) Medical Center between December 2018 and April 2021. Patients of all cancer grades and stages were included in the study. Additional study inclusion criteria included having an active outpatient prescription for 1 of 3 PARP inhibitors (olaparib, rucaparib, or niraparib) written by a UNC gynecology/oncology physician and having a documented BRCA1/2 mutation. BRCA1/2 mutations were identified by one of the following genetic testing vendors: Ambry Genetics, Foundation Medicine, Invitae, Myriad Genetics, Strata Oncology, or Tempus. The patients’ characteristics, tumor pathology, genetic testing, molecular markers, and treatment characteristics were collected.
Patients were excluded from the study if they had clear-cell or mucinous epithelial ovarian cancer, had BRCA wild-type mutation status, were receiving PARP inhibitors as part of a clinical trial, were receiving PARP inhibitors in combination with other therapies, or were receiving PARP inhibitor therapy for the treatment of cancer. Patients were also excluded from the final analysis if the PARP inhibitor prescription was never filled because of financial restrictions, personal preference, disease progression, or transition into hospice care, or if the patient had received care at an outside institution for the duration of their treatment after the initial prescription was sent.
The primary outcome was the duration of PARP inhibitor maintenance therapy as determined by the start date of therapy and the date of therapy discontinuation. The decision was made to characterize our patients with duration of therapy to reduce errors involved in retrospectively interpreting serial imaging scans and to assess therapeutic benefit overall. The dates were collected from notes within the EHR and were confirmed with prescription fill histories. In addition, if patients participated in more than 1 trial of a PARP inhibitor during the study period, the total duration of PARP inhibitor therapy was reflected by calculating the sum of the 2 trial durations.
The secondary study outcomes included the rate of dose interruptions, reductions, and discontinuation resulting from adverse events, the rates of Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 grade 3 and 4 adverse events, and the rate of LOH testing.23 In the patients who were included in 1 PARP inhibitor trial, each PARP inhibitor was analyzed separately for adverse events. The documentation of follow-up visits was reviewed to identify the incidence and CTCAE grade of adverse events and to determine the reasons for therapy interruptions and/or discontinuations. Treatment interruptions were defined as a delay in treatment of 5 days or longer.
Statistical Analysis
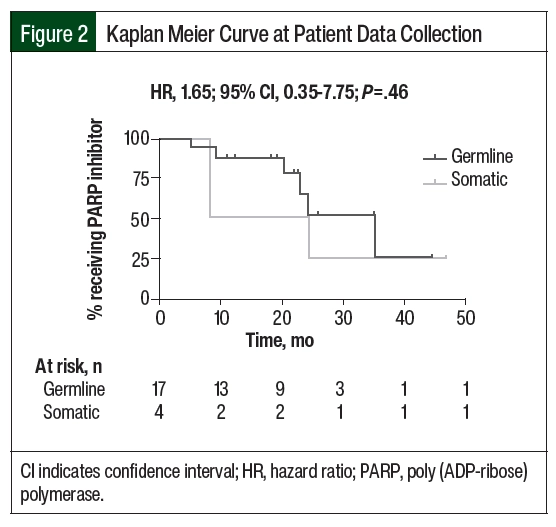
The patients were stratified by germline and somatic BRCA mutation status. Descriptive statistics were used to assess the patients’ demographics and the secondary clinical outcomes. The primary outcome of duration of PARP inhibitor maintenance therapy was analyzed using a Cox regression model. A Kaplan Meier curve was constructed to further assess the differences in the duration of PARP inhibitor therapy between the 2 groups. A hazard ratio (HR) with a 95% confidence interval (CI) was calculated and reported. Of note, the Kaplan Meier curve accounted for patients who were still receiving therapy with PARP inhibitors at the time of data collection. Statistical analyses were conducted using R software version 4.1.2 (R Foundation for Statistical Computing; Vienna, Austria).
Results
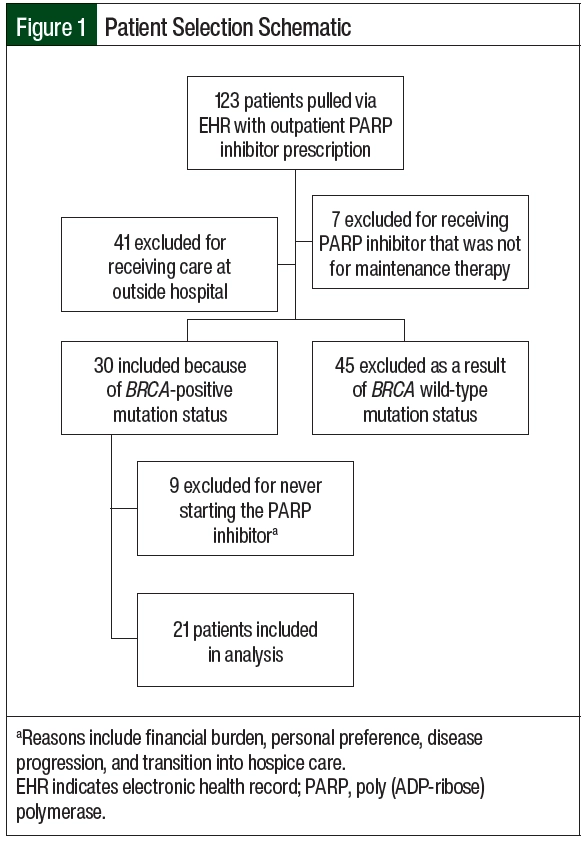
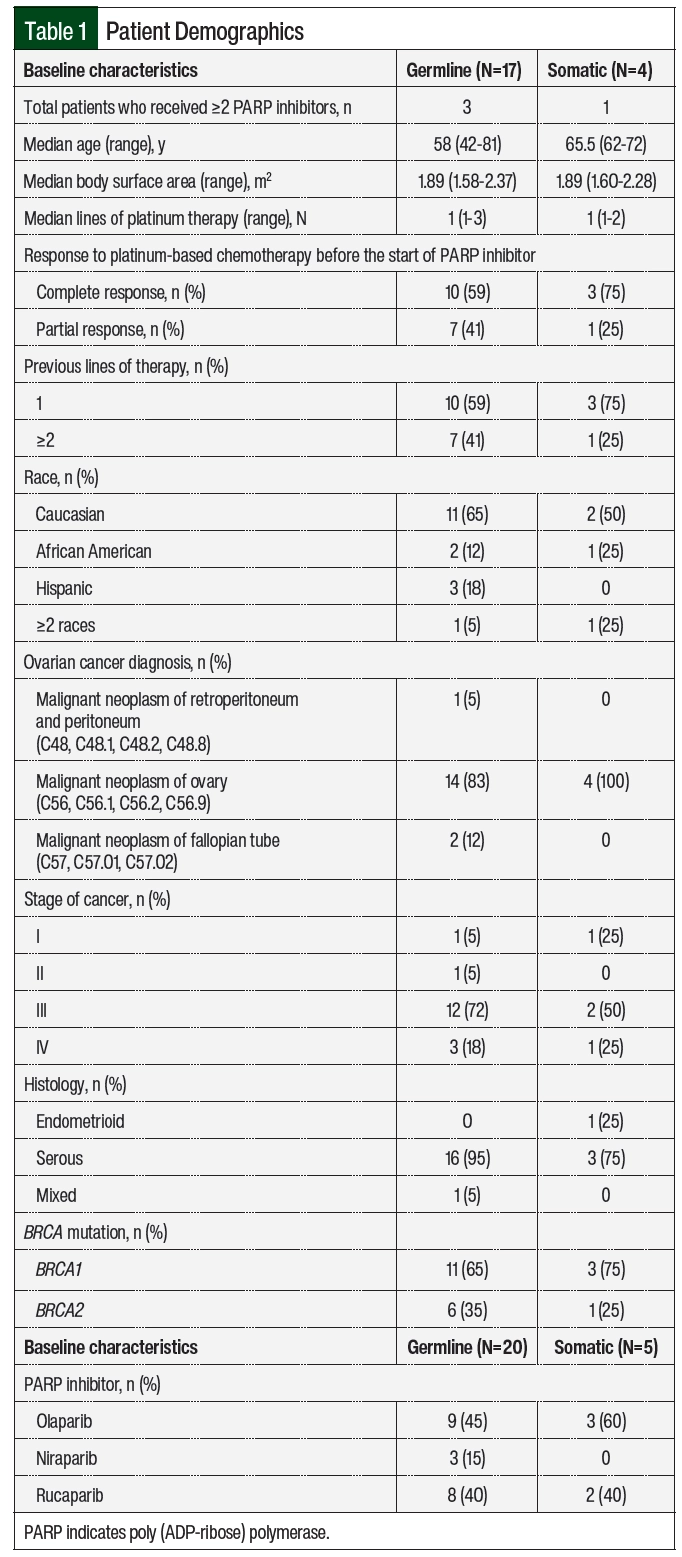
In all, 123 patients had an outpatient prescription for PARP inhibitors during the study period (Figure 1). A total of 102 patients were excluded from the study (Figure 1), leaving 21 patients who met the study eligibility criteria (germline, n=17; somatic, n=4), with 4 patients receiving more than 1 PARP inhibitor during the study period (germline, n=3; somatic, n=1). The most common reasons for study exclusion were that the patient was primarily managed at an outside institution (n=41) or that the patient did not have a BRCA mutation (n=45). Of the 21 included patients, 17 (81%) patients had a germline mutation, of which 11 (65%) were BRCA1 and 6 (35%) were BRCA2 (Table 1). The remaining 4 (19%) patients had a somatic mutation, of which 3 (75%) were BRCA1 and 1 (25%) was BRCA2. The 2 patient groups were demographically similar. The median ages in the germline and somatic BRCA mutation groups were 58 and 65.5 years, respectively. The majority of patients in the study had stage III disease and had received a median of 1 line of platinum-based chemotherapy. The most frequently received PARP inhibitor for maintenance therapy was olaparib (12/25; 48%).
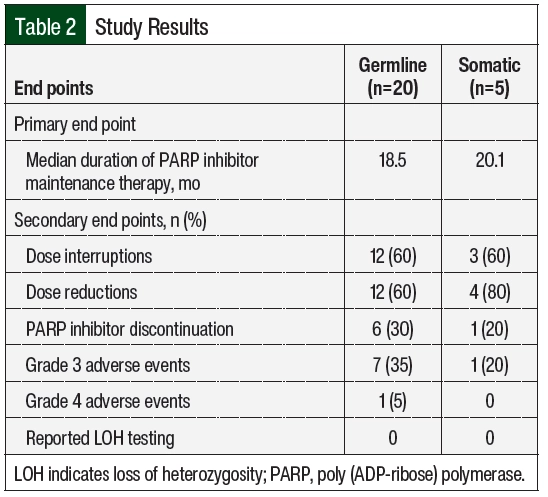
The median duration of PARP inhibitor therapy was 18.5 months in the patients with germline BRCA mutation and 20.1 months in the patients with somatic BRCA mutation (HR, 1.65; 95% CI, 0.35-7.75; P=.46; Figure 2). There was no significant difference in the duration of therapy between the 2 groups. In all, 11 (64%) patients with germline BRCA mutation and 1 (25%) patient with somatic BRCA mutation remained on PARP inhibitor treatment at the time of data collection. In the remaining 6 patients with germline BRCA mutation, the reasons for PARP inhibitor discontinuation included disease progression (17%), completion of therapy after 2 years (33%), and adverse events (50%). In the remaining 3 patients with somatic BRCA mutation, the reasons for discontinuation included progression of disease (66%) and the completion of therapy after 2 years (33%). For the 4 patients (3 with germline BRCA mutation and 1 with somatic BRCA mutation) who had 2 consecutive trials of PARP inhibitor therapy, the reason for discontinuation of the first therapy was adverse events (100%). A total of 4 patients received ≥2 PARP inhibitors during the study period, with rucaparib being the most received second-line PARP inhibitor (3 of the 4 patients). Of the included patients, 7 (33%) were receiving maintenance therapy with a PARP inhibitor beyond 2 years and 2 (10%) were receiving therapy beyond 3 years.
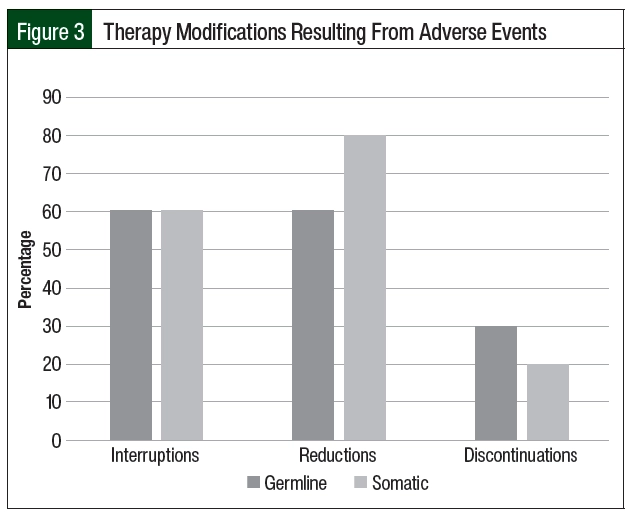
Of the 25 unique cases of patients who received PARP inhibitors, 15 (60%) required dose interruptions as a result of adverse events during the study period (Table 2). The number of dose interruptions was 12 in the 20 cases among the patients with germline BRCA mutation versus 3 in the 5 cases among the patients with somatic BRCA mutation. Most of the patients with germline and somatic BRCA mutations had dose reductions during the study period, including 12 of the 25 (60%) patients with germline BRCA mutations and 4 of the 5 (80%) with somatic BRCA mutation (Table 2, Figure 3). The most common reason for dose interruptions and reductions was anemia. Although most patients in both groups had dose reductions and interruptions of PARP inhibitor therapy, few patients ultimately required treatment discontinuation as a result of adverse events (6 of the 20 [30%] patients with germline BRCA mutation vs 1 of the 5 [20%] patients with somatic BRCA mutation). The most common reason for the discontinuation of PARP inhibitor therapy was anemia (2 of 5 patients). The incidence of grade 3 CTCAE adverse events was similar to previous reports (35% of patients with germline BRCA mutation vs 20% of patients with somatic BRCA mutation).18,21,24 One patient with germline BRCA mutation had a grade 4 CTCAE adverse event (ie, thrombocytopenia). None of the 21 patients in the study had LOH.
Discussion
The results of this study showed a similar duration of therapy with PARP inhibitors in the maintenance setting between patients with ovarian cancer with germline and somatic BRCA mutations, with similar rates of PARP inhibitor discontinuation as a result of adverse events. Of note, patients in the somatic BRCA mutation group had numerically longer median durations of therapy, which is surprising given the lower prevalence of other markers of HRD in patients with somatic BRCA mutation–positive ovarian cancer.9 PARP inhibitor use in both cohorts was well tolerated, with the majority of patients requiring treatment reductions and/or interruptions, but not requiring treatment discontinuation. The early results of the ORZORA study assessing maintenance treatment with olaparib in patients with platinum-sensitive relapsed ovarian cancer have similarly demonstrated consistent clinical activity of olaparib use between patients with germline BRCA mutation and those with somatic BRCA mutation.25
Limitations
Our study is limited by its retrospective nature and the small number of patients who met the enrollment criteria. We used duration of maintenance therapy as our primary end point to remove bias or error with the interpretation of serial imaging scans, but this somewhat limits our ability to directly compare our data with those of other studies that used PFS as their primary outcome. In addition, using the duration of germline or somatic BRCA mutations can be difficult to interpret as a surrogate for PFS given that patients can complete therapy after 2 or 3 years in the primary maintenance setting. When this study was designed, we did not yet have the reassuring PFS follow-up data from the SOLO-1 trial to confirm the durable response beyond the 2-year completion point.18 With PFS benefit still present at 5 years, it is now standard practice to discontinue PARP inhibitor therapy after 2 years for olaparib or 3 years for niraparib.
None of the patients in our study underwent testing for LOH or HRD status. This may be because of the lack of data and clear recommendations in favor of doing this type of testing during the study period. Although this outcome is disappointing, it underscores the need to continue to evaluate patient populations for relevant biomarkers of drug benefit as data on targeted agents evolve. Real-world patient cohorts that have been evaluated for these biomarkers can provide insights into how to optimize care beyond what was attainable with data from clinical trials. The implementation of expanded testing to include other HRD markers is limited by inconsistencies observed between the different assays and clinical study designs. Various laboratories are developing different approaches to reporting HRD scores, which may include BRCA mutation status, LOH, and other markers. Others are basing such a score primarily on LOH.10 Further validation of these scores and their wider use in patients will help our understanding of ovarian cancer and advance our use of PARP inhibitors beyond using BRCA mutation status alone.
These data add to the body of literature striving to define biomarkers to predict benefit from PARP inhibitors. A future consideration may be to expand biomarker testing in patients with BRCA-positive and BRCA wild-type mutations. This may help improve outcomes with PARP inhibitors by identifying the patients who may benefit from evolving combination therapy approaches or who may benefit more from novel treatment approaches in the pipeline.26 Our study involved intensive chart review to identify patients who had BRCA mutations. Organizations should continue to strive to include molecular information on cancer as discrete data to allow for the progressive investigation of biomarkers in real-world populations and to provide a nimble approach as new biomarkers are identified and integrated into clinical practice.
Conclusion
Although patients with germline or somatic BRCA mutation–positive ovarian cancers demonstrated similar durations of treatment with PARP inhibitor maintenance, there remains a paucity of data. Additional studies in larger patient populations are recommended to further investigate the use of additional biomarkers, such as LOH, on the efficacy of PARP inhibitors. The results from larger population studies could have an immense impact on maintenance therapy for patients with ovarian cancer.
References
- National Cancer Institute SEER Program. Cancer stat facts: ovarian cancer. Accessed January 18, 2024 . https://seer.cancer.gov/statfacts/html/ovary.html
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66-71.
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402-2416.
- Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654-2663. Erratum in: J Clin Oncol. 2012 Nov 20;30(33):4180
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Konstantinopoulos PA, Norquist B, Lacchetti C, et al. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:1222-1245.
- Hodgson DR, Brown JS, Dearden SP, et al. Concordance of BRCA mutation detection in tumor versus blood, and frequency of bi-allelic loss of BRCA in tumors from patients in the phase III SOLO2 trial. Gynecol Oncol. 2021;163:563-568.
- Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571:576-579. Erratum in: Nature. 2020;577:E1.
- Miller RE, Leary A, Scott CL, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol. 2020;31:1606-1622.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Version 1.2024. Accessed January 18, 2024. www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
- Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776-1782.
- Jiang X, Li X, Li W, et al. PARP inhibitors in ovarian cancer: sensitivity prediction and resistance mechanisms. J Cell Mol Med. 2019;23:2303-2313.
- Mittica G, Ghisoni E, Giannone G, et al. PARP inhibitors in ovarian cancer. Recent Pat Anticancer Drug Discov. 2018;13:392-410.
- Walsh CS. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137:343-350.
- De Lorenzo SB, Patel AG, Hurley RM, Kaufmann SH. The elephant and the blind men: making sense of PARP inhibitors in homologous recombination deficient tumor cells. Front Oncol. 2013;3:228.
- AstraZeneca. Lyparza approved by the US food and drug administration for the treatment of advanced ovarian cancer in patients with germline BRCA-mutations. December 19, 2014. Accessed January 24, 2024. www.astrazeneca.com/media-centre/press-releases/2014/lynparza-approved-us-fda-brca-mutated-ovarian-cancer-treatment-19122014.html#
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428.
- Jiang X, Li W, Li X, et al. Current status and future prospects of PARP inhibitor clinical trials in ovarian cancer. Cancer Manag Res. 2019;11:4371-4390.
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Mohyuddin GR, Aziz M, Britt A, et al. Similar response rates and survival with PARP inhibitors for patients with solid tumors harboring somatic versus germline BRCA mutations: a meta-analysis and systematic review. BMC Cancer. 2020;20:507.
- National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. November 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Pignata S, Oza AM, Hall G, et al. Maintenance olaparib in patients (pts) with platinum-sensitive relapsed ovarian cancer (PSROC) by somatic (s) or germline (g) BRCA and other homologous recombination repair (HRR) gene mutation status: overall survival (OS) results from the ORZORA study. J Clin Oncol. 2022;40(16 suppl):Abstract 5519.
- Miller RE, El-Shakankery KH, Lee JY. PARP inhibitors in ovarian cancer: overcoming resistance with combination strategies. J Gynecol Oncol. 2022;33:e44.