Acute myeloid leukemia (AML) is a hematologic malignancy characterized by a proliferation of myeloblasts in the bone marrow, peripheral blood, and/or tissues (extramedullary disease).1 AML most frequently affects the elderly (aged ≥65 years) population, with a median age at diagnosis of 69 years.2 The treatment for AML is divided into induction and consolidation chemotherapies.3 The intensity of induction chemotherapy depends on the patient’s ability to tolerate chemotherapy based on performance status, age, and preexisting comorbidities. The National Comprehensive Cancer Network (NCCN) guidelines recommend intensive induction chemotherapy for patients aged <60 years who are fit to tolerate chemotherapy (ie, anthracycline such as daunorubicin or idarubicin with cytarabine).3 For select patients who are not candidates for intensive induction, the NCCN guidelines recommend treatment with a hypomethylating agent such as azacitidine or decitabine alone or in combination with venetoclax.3
The FDA approved venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine for the treatment of newly diagnosed AML in patients aged ≥75 years, or for patients with comorbidities that preclude the use of intensive induction chemotherapy regimens.4 In addition, the FDA and NCCN guidelines recommend a 3- or 4-day ramp-up for venetoclax, depending on the combination (100 mg on day 1, 200 mg on day 2, and 400 mg on day 3 and beyond when combined with azacitidine or decitabine, and the same regimen when combined with low-dose cytarabine, with the exception of 600 mg starting on day 4 and beyond). Venetoclax dose ramp-up is used to reduce the incidence of tumor lysis syndrome (TLS) along with waiting to start venetoclax until the white blood cell (WBC) count is <25 k/mcL.3,4 TLS is an oncology emergency caused by the rapid lysis of tumor cells, which can occur after the initiation of chemotherapy; patients with hematologic malignancies, such as AML, have a high risk for TLS.5 TLS can cause laboratory abnormalities, such as hyperkalemia, hyperuricemia, hyperphosphatemia, and hypocalcemia, as well as clinical complications, including renal insufficiency, seizures, cardiac arrhythmias, or death.5
There are limited published data describing the incidence of TLS in patients with AML who initiated treatment with venetoclax without dose ramp-up in combination with a hypomethylating agent. At Allegheny Health Network Cancer Institute (AHNCI), the clinical practice is to start venetoclax at the full dose (omitting the 3-day ramp-up) once the WBC count is <25 k/mcL when used for the treatment of AML. The purpose of this study was to determine our institution’s incidence of clinical and laboratory TLS in patients with AML who initiated treatment with venetoclax, without dose ramp-up, in combination with a hypomethylating agent.
Methods
This retrospective, single-arm study included patients aged 18 to 89 years with newly diagnosed or relapsed or refractory AML who received treatment with venetoclax in combination with a hypomethylating agent (azacitidine or decitabine) at AHNCI, West Penn Hospital, from February 2019 to September 2021. The exclusion criteria were having a diagnosis other than AML, being incarcerated, and pregnancy. The patients received azacitidine 100 mg/m2 intravenously or subcutaneously every 24 hours on days 1 to 5 of a 28-day cycle or decitabine 20 mg/m2 intravenously every 24 hours on days 1 to 5 or days 1 to 10 of a 28-day cycle in combination with venetoclax 400 mg orally every 24 hours.3,4,6,7 The azacitidine dosing schedule of 100 mg/m2 given more than 5 days (cumulative dose, 500 mg/m2) is standard dosing within our institution, which differs slightly from the dosing of azacitidine in the published literature of 75 mg/m2 given more than 7 days (cumulative dose, 525 mg/m2).6 Venetoclax was dose adjusted at 50% of the target dose (200 mg) for fluconazole and at a 75% dose reduction (100 mg) for posaconazole and voriconazole. If patients were TP53 mutation–positive, treatment with decitabine was extended to a 10-day duration.7 All patients were admitted to the hospital for the first cycle of chemotherapy.
The data that were collected from the electronic health records included the patients’ demographics, hypomethylating agent dosing, venetoclax dosing, bone marrow biopsy reports, TLS laboratory values at baseline and the highest within 72 hours of treatment initiation (serum creatinine, potassium, corrected calcium, phosphorous, uric acid, and WBC count), the clinical TLS symptoms at baseline and within 72 hours of treatment initiation (renal insufficiency, dysrhythmia, seizure, and death), rasburicase administration, and concomitant azole antifungal.
The primary end point was to evaluate the incidence of laboratory and clinical TLS among the patients who initiated treatment with venetoclax without dose ramp-up in combination with a hypomethylating agent. Clinical and laboratory TLS were defined based on Cairo-Bishop definition within 72 hours of starting chemotherapy.5 The secondary end points evaluated the incidence of adverse events (alanine aminotransferase [ALT], aspartate aminotransferase [AST], blood bilirubin, diarrhea, nausea, peripheral edema, and vomiting) during cycle 1, and the incidence of adverse events with interacting azole antifungals (fluconazole, isavuconazole, posaconazole, and voriconazole). The adverse events were graded using the Common Terminology Criteria for Adverse Events version 5.0.8 This study was approved by the local institutional review board (IRB 2021-161).
The patient demographic and characteristic data were analyzed by frequency (percentage) for categorical variables and mean (standard deviation) or median (interquartile range) for continuous variables, as appropriate.
Results
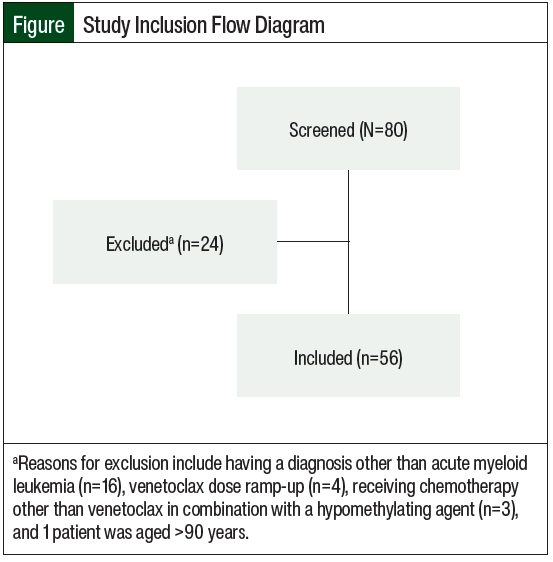
A total of 80 patients were screened for inclusion in the study. A total of 24 patients were excluded because they had a diagnosis other than AML, had a dose ramp-up of venetoclax, received a chemotherapy regimen other than venetoclax in combination with a hypomethylating agent, or were aged ≥90 years. A total of 56 patients were included in the study (Figure).
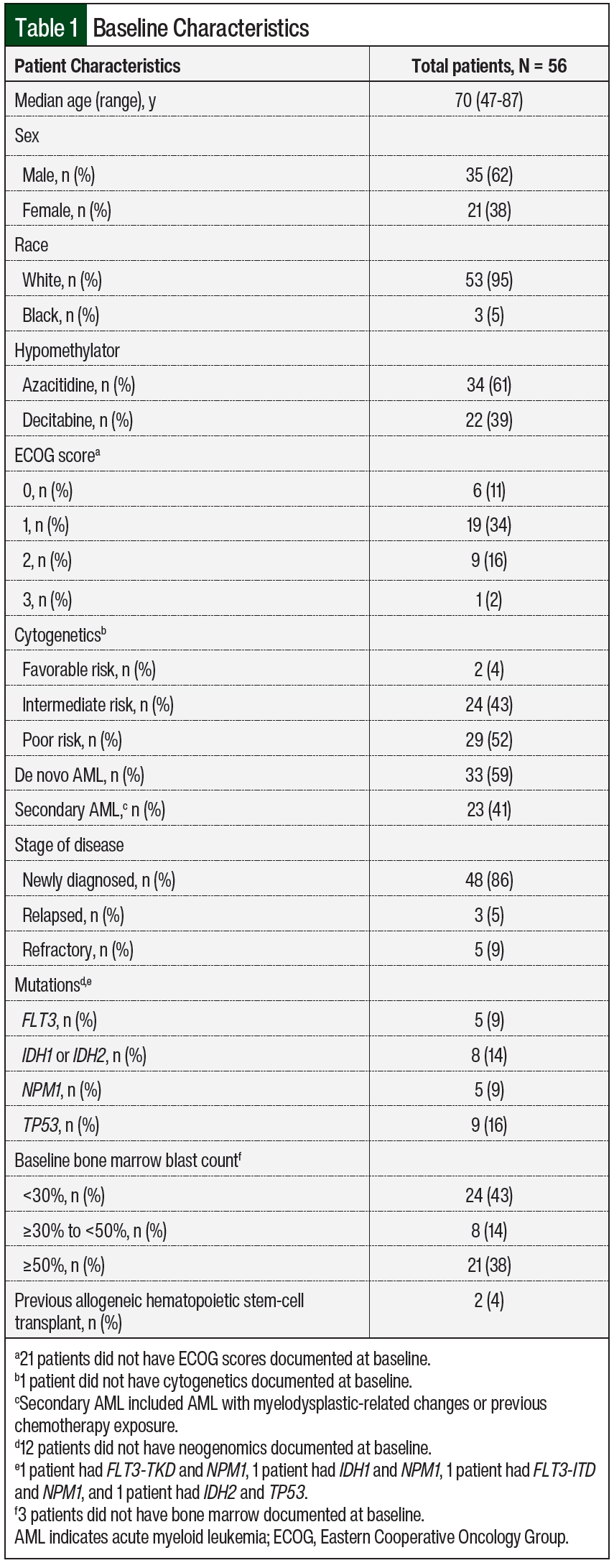
Most patients were men and had newly diagnosed AML with intermediate- to poor-risk cytogenetics (Table 1). The majority of patients received azacitidine in combination with venetoclax (n=34; 61%). Some patients were cytoreduced with hydroxyurea and/or leukapheresis before starting treatment with venetoclax plus a hypomethylating agent (n=6; 11%). A total of 9 (16%) patients received rasburicase.
A total of 3 (6%) patients had TLS, of whom 2 had laboratory TLS (hyperuricemia and hyperphosphatemia) and 1 had clinical TLS (hyperuricemia and renal insufficiency, not requiring hemodialysis). Of the patients who had TLS, 1 patient received treatment with rasburicase. Patient 1 was receiving decitabine with venetoclax and posaconazole. This patient had a WBC count of 30.6 k/mcL with circulating peripheral blasts of 18 k/mcL, and then was further cytoreduced with hydroxyurea to a WBC count of 0.68 k/mcL before day 1 of chemotherapy. In addition, this same patient had new renal insufficiency as part of their clinical TLS symptoms. Patient 2 was receiving azacitidine with venetoclax and fluconazole. This patient had a WBC count of 3 k/mcL with circulating peripheral blasts of 0.2 k/mcL and did not need further cytoreduction before chemotherapy. Patient 2 had stage 3 chronic kidney disease at baseline and received rasburicase. Patient 3 was receiving azacitidine with venetoclax and fluconazole. This patient had a WBC count of 12.9 k/mcL with circulating peripheral blasts of 7 k/mcL and did not need further cytoreduction.
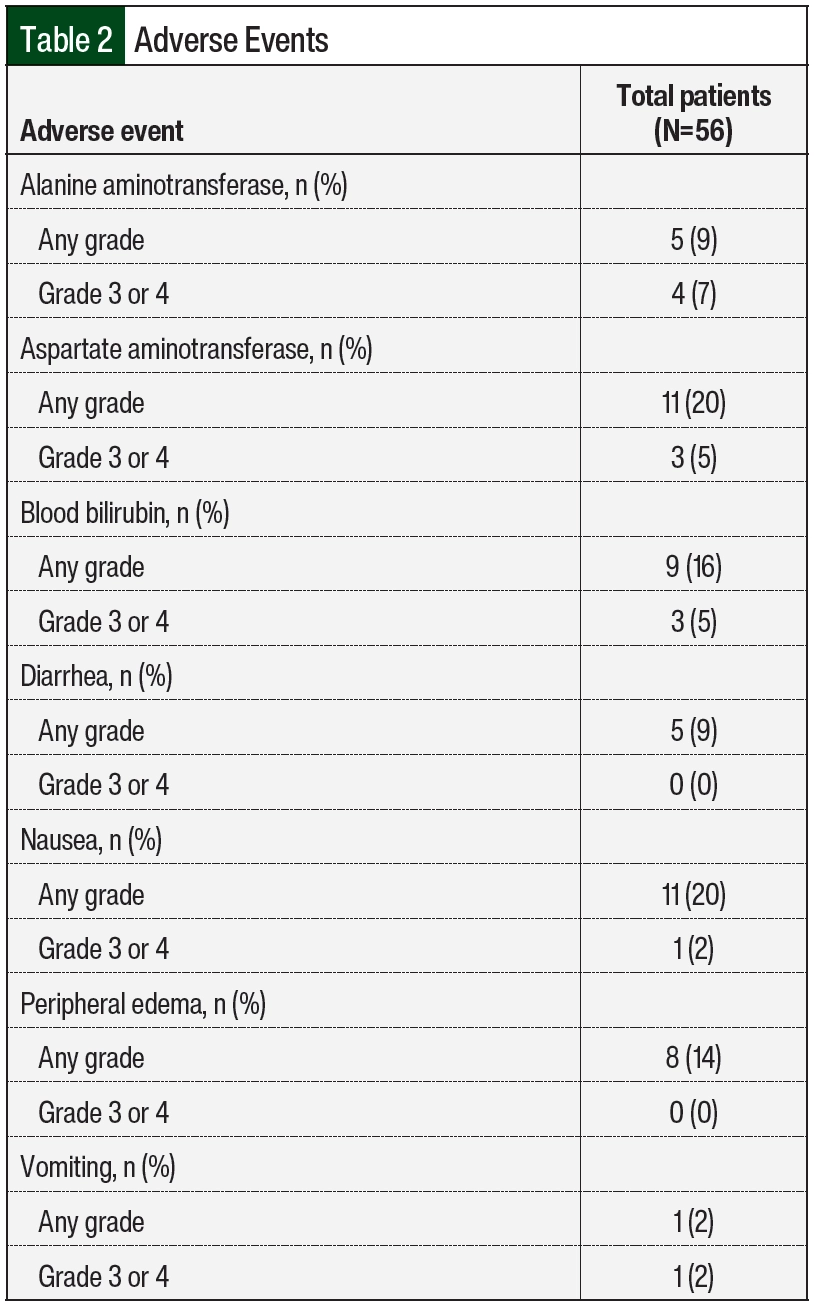
A total of 25 (45%) patients had an adverse event, and 13 (23%) of these 25 patients had more than 1 adverse event (Table 2). The most common adverse events during treatment were increased AST and nausea. Increased AST was observed in 11 (20%) patients, with 3 (5%) patients having a grade 3 or 4 AST elevation. In all, 9 (16%) patients had an elevation in at least 2 of the following: AST, ALT, or blood bilirubin. Of the 12 patients who had any-grade AST, ALT, or bilirubin elevation, 11 were receiving an azole antifungal. Nausea was observed in 11 (20%) patients, with 1 patient having a grade 3 or 4 event.
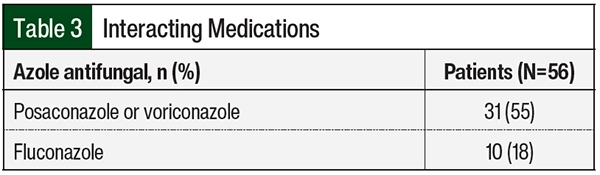
Of the 56 patients in the study, 41 (73%) were receiving fluconazole, voriconazole, or posaconazole that affected the starting dose of venetoclax (Table 3). Venetoclax was appropriately dose adjusted to account for these interactions. Of the 3 patients who had TLS, 1 patient was receiving posaconazole and 2 patients were receiving fluconazole. The 2 patients who were receiving fluconazole changed to treatment with posaconazole once a diagnosis of AML was confirmed; however, they already received 1 dose of fluconazole with their chemotherapy.
Discussion
This retrospective study of patients with AML resulted in an incidence rate of 6% for TLS when omitting the venetoclax 3-day dose ramp-up combined with a hypomethylating agent. Two patients had laboratory TLS (hyperuricemia and hyperphosphatemia). One patient had clinical TLS (hyperuricemia and renal insufficiency, not requiring hemodialysis). The NCCN’s recommendation for cycle-1 venetoclax is 100 mg on day 1, 200 mg on day 2, and 400 mg on day 3 and thereafter.3 Our institution’s practice is that once the WBC count is <25 k/mcL, venetoclax is initiated at a target dose of 400 mg. Because our institution’s clinical practice is to omit the venetoclax dose ramp-up, we compared our incidence result for TLS with that in the literature.
After a review of the currently available literature, there are few published real-world articles that describe the incidence of TLS with venetoclax when omitting the dose ramp-up for AML treatment. When a venetoclax dose ramp-up was used, Dinardo and colleagues reported a TLS rate of 1% in the azacitidine plus venetoclax group and in none of the patients in the control group.9 Using a 10-day decitabine regimen in combination with a venetoclax dose ramp-up, Dinardo and colleagues reported rates of 2% and 1%, respectively, for grades 3 and grade 4 TLS.7 In their phase 1b dose-escalation and -expansion study of venetoclax in combination with a hypomethylating agent, Dinardo and colleagues reported that no TLS was observed.10 Real-world data on the incidence of TLS in patients who received venetoclax in a 3-day dose ramp-up in 10 centers across Israel showed that clinical and laboratory TLS was reported in 6 (4.7%) of 127 patients who received a hypomethylating agent and venetoclax.11 Shahswar and colleagues reported an incidence of 12% for TLS in newly diagnosed patients with relapsed or refractory AML who received venetoclax without a dose ramp-up in combination with a hypomethylating agent or low-dose cytarabine.12 This is the only study we could find that reports TLS without the dose ramp-up of venetoclax.
Our study’s incidence of TLS was lower than the study without dose ramp-up (6% vs 12%, respectively).12 However, our study’s results showed a slightly higher rate of TLS than in those studies with a dose ramp-up (6% vs 1%-4.7%, respectively).7,11 Thus, we conclude that omitting the venetoclax dose ramp-up in combination with a hypomethylating agent is safe for treatment cycle 1. For our patient population, all patients received appropriate tumor lysis prophylaxis with hydration, allopurinol, and rasburicase per the AHNCI guidelines. Our institution’s TLS algorithm recommends that patients with low-risk TLS receive hydration only with normal saline at a rate of 75 to 150 mL per hour based on patient tolerability, that patients with intermediate-risk TLS receive hydration with allopurinol 300 mg orally daily and that allopurinol may be renally adjusted, and that patients with high-risk TLS receive hydration with allopurinol and rasburicase 3 mg or 6 mg. For patients with baseline uric acid of >10 mg/dL and a WBC count of >50 k/mcL or serum creatinine >1.5 mg/dL, rasburicase 6 mg is administered once.
All of our patients were admitted in the hospital for close monitoring during treatment cycle 1 and were then transitioned to outpatient care for cycles 2 and later. A total of 6 patients started treatment with venetoclax after cycle 1 because of insurance issues when venetoclax was not part of the hospital formulary; therefore, they were excluded from the primary end point determining TLS because a later venetoclax start date could be a confounding variable. In all, 6 (11%) patients were cytoreduced using hydroxyurea and/or leukapheresis before starting treatment with venetoclax. Explanations for our lower incidence of TLS versus in the study by Shahswar and colleagues12 include that most of our patient population had a WBC count of <25 k/mcL on presentation and for those patients with a WBC count of ≥25 k/mcL, we further cytoreduced with hydroxyurea and/or leukapheresis. Categorizing our patient population based on TLS risk, 40 (80%) patients had a low risk for TLS, 7 (14%) patients had an intermediate risk, and 3 (6%) patients had a high risk.5
The chemotherapy regimen of venetoclax in combination with a hypomethylating agent was well tolerated, with few patients having grade 3 or 4 adverse events. The most common adverse events were increased AST and nausea. A total of 43 (77%) patients were receiving an azole antifungal, with venetoclax, posaconazole, and voriconazole being the most common. In all, 10 patients were receiving fluconazole even though the recommended azole antifungal for patients with AML who are receiving a hypomethylating agent with venetoclax is posaconazole. The reasons for this choice were lack of confirmed AML diagnosis early on during hospitalization so treatment with fluconazole was started for patients who presented with neutropenia and then switched to treatment with posaconazole once a diagnosis was confirmed; cost, because some patients’ prescription insurance did not cover posaconazole nor voriconazole or the copay was unaffordable; or significant interacting medications precluding the addition of a strong cytochrome 3A4 inhibitor. Posaconazole azole antifungal is the preferred agent in these patients with AML; however, sometimes this may not be possible in clinical practice.
Limitations
The limitations of this study design include being a single-center, retrospective study with a small sample size. Some baseline patient demographics were missing because of a lack of documentation in the electronic health records, such as performance status, incomplete diagnostic workup as a result of poor bone marrow aspirate quality, or the initial workup was incomplete because it was done at an outside institution before the patient was transferred to AHNCI (missing cytogenetics or neogenomics in Table 1). A total of 3 patients did not have bone marrow documented at baseline because disease progression was determined by peripheral blast count or extramedullary disease (Table 1).
Of the 12 patients receiving an antifungal medication, 11 had any degree of ALT, AST, or bilirubin elevation. Azole antifungals are well known to cause elevations in ALT, AST, and/or bilirubin along with venetoclax (increased serum AST of 53%).4 However; because of a lack of power for these adverse events, it is difficult to determine whether venetoclax or the azole antifungal caused the adverse events, which is another limitation of this study.
Conclusion
Before this study, there was limited literature omitting venetoclax dose ramp-up when combined with a hypomethylating agent in patients with AML. Our study adds to the scarce information that is currently available in the literature. We demonstrated that omitting venetoclax dose ramp-up when combined with a hypomethylating agent in patients with AML can be safely done during hospitalization in treatment cycle 1. These data may support the use of omitting venetoclax dose ramp-up for patients with AML at other institutions.
Author Disclosure Statement
Dr Fazal is on the Speaker’s Bureau of BMS, Karyophann, Phannaessentia, Taiho, Blueprint, Pfizer, Incyte, Janssen, GSK, Takeda, Stemline, Omeros, Sanofi, Novartis, Servier, Gilead, and CTI biopharma, and is a consultant to BMS, Taiho, Blueprint, Incyte, GSK, Takeda, Stemline, Omeros, Sanofi, Novartis, Servier, Gilead, and CTI biopharma; Dr Gray, Dr Ursu, Dr Alhousani, and Ms Caruso have no conflicts of interest to report.
References
- Saultz JN, Garzon R. Acute myeloid leukemia: a concise review. J Clin Med. 2016;5:33.
- National Cancer Institute. SEER cancer stat facts: leukemia-acute myeloid leukemia. Accessed March 6, 2024. https://seer.cancer.gov/statfacts/html/amyl.html
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): acute myeloid leukemia. Version 6.2023. October 24, 2023. Accessed March 6, 2024. www.nccn.org/professionals/physician_gls/pdf/aml.pdf
- Venclexta (venetoclax) tablets, for oral use [prescribing information]. AbbVie; June 2022. Accessed March 12, 2024. www.rxabbvie.com/pdf/venclexta.pdf
- Belay Y, Yirdaw K, Enawgaw B. Tumor lysis syndrome in patients with hematological malignancies. J Oncol. 2017;2017:9684909.
- Haq B, Rossetti JM, Kramer W, et al. Response and tolerability of a 5-day azacytidine schedule in patients with myelodysplastic syndromes. J Clin Oncol. 2006;24(18 suppl):16532.
- DiNardo CD, Maiti A, Rausch CR, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7:e724-e736.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. November 27, 2017. Accessed December 1, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617-629.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7-17.
- Wolach O, Levi I, Lavie D, et al. Real world prospective observational multicenter trial of venetoclax-based therapy for patients with AML reveals unique patterns of patient selection and treatment utilization - revive study. Blood. 2021;138(suppl 1):1246-1248.
- Shahswar R, Beutel G, Gabdoulline R, et al. Risk of tumor lysis syndrome in patients with acute myeloid leukemia treated with venetoclax-containing regimens without dose ramp-up. Ann Hematol. 2021;100:595-599.