In an effort to improve response rates to chemotherapy, agents often are combined to give a so-called twoprong attack against tumor cells. This is the basis for combining chemotherapy agents with different mechanisms of action. However, chemotherapy agents are subject to the rules of pharmacokinetics and thus have the potential for drug interactions. A common misconception is that because the agents have been tested as part of a chemotherapy regimen, they are safe and effective. The problem is that many phase 1 and 2 studies of a particular regimen do not give specifics about the order in which these agents were administered. Several chemotherapy agents (eg, doxorubicin, docetaxel, paclitaxel, and others) are extensively metabolized through the cytochrome P450 pathway, and many chemotherapy agents (eg, taxanes and platinum agents) have high degrees of protein binding. In addition, many chemotherapy agents have cell cycle–specific mechanisms of action that may increase the cytotoxicity or antagonize the mechanism of the second agent.1 In some situations, the order of administration may dictate whether a particular effect or side effect is encountered based on the principles of pharmacokinetics and pharmacodynamics. Perhaps the most well known is the interaction between cisplatin and paclitaxel.2 When cisplatin precedes paclitaxel, profound and prolonged neutropenia may occur. This can delay the patient from receiving chemotherapy as prescribed. When the sequence is reversed (ie, paclitaxel before cisplatin), this detrimental side effect is diminished without negating efficacy.2
These concerns present real situations and lead to drug information questions that are often asked in a chemotherapy infusion center. Although there are extensive published data for some agents, like the taxanes, about drug interactions and sequencing, the data often can be hard to find, especially for less common or newer agents.1-5 In addition, drug information resources extrapolate published data from single agents to all agents in the same class, recommending a sequence that may not be proved clinically. An example is extrapolating the data on cisplatin and paclitaxel to imply the same interaction holds true between oxaliplatin and paclitaxel or carboplatin and docetaxel.
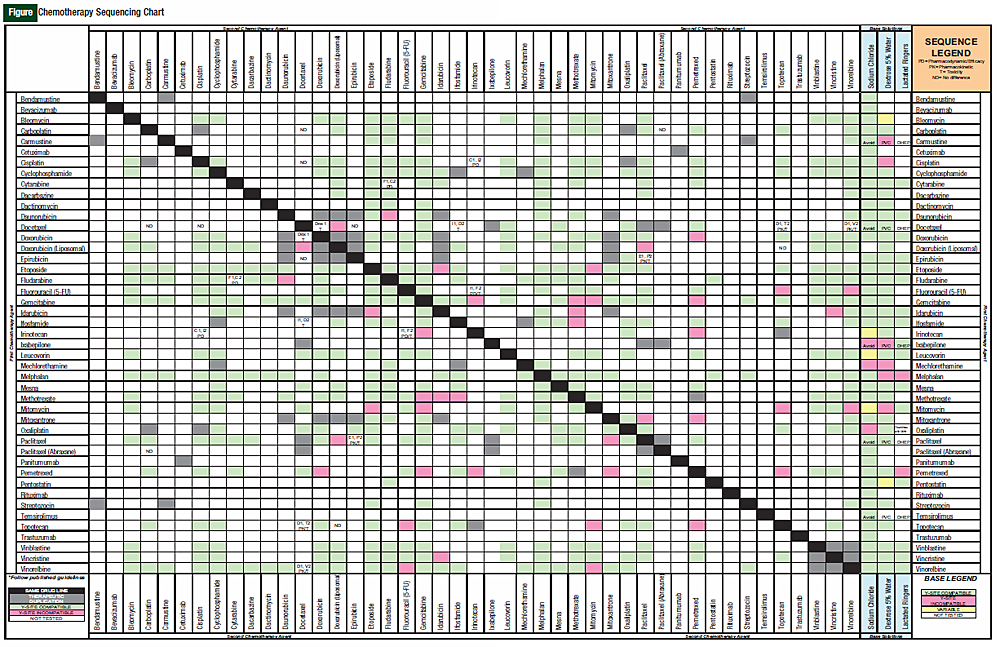
We conducted a thorough review of the literature to examine the data that have been published supporting same-day chemotherapy administration sequences. From these data, we created a chemotherapy administration sequence chart to aid nurses and pharmacists in identifying whether there is a preferred sequence to be used only for agents given on the same day. We then superimposed this chart on a chemotherapy compatibility chart to make a new tool for use in our infusion centers.
Methods
First, a list of all intravenous chemotherapy and monoclonal antibodies commonly used in combination regimens was created. Then, Micromedex Solutions’ IV Index with Trissle’s compatibility tool was used to assess whether medications were physically compatible via Ysite coinfusion. In addition, base solution compatibilities (lactated Ringers, normal saline, and dextrose 5% in water) for individual chemotherapy agents was evaluated. These steps were performed because they were easy to incorporate into the finalized chart, and it helped to combine existing compatibility charts with the sequencing chart into a single tool.
For the sequencing chart, the administration sequence of the original studies on published regimens was evaluated. Any chemotherapy agent that is not traditionally given on the same day as another agent was excluded from the chart. Lexi-Comp and Micromedex were evaluated, as standards for drug information resources, for recommended sequence specifics in the administration and drug interaction sections. Finally, a thorough PubMed search was conducted using keyword searches including any combination of the drug name with “administration,” “sequencing,” or “interactions.”
After the literature base had been established, studies were evaluated to determine whether they were clinically applicable. Studies were included if they were conducted in humans and evaluated both the forward and reverse sequence of administration. Review articles were also considered if they provided human clinical trial data. All other studies were excluded. These exclusion criteria were selected because those types of studies could not be extrapolated to in vivo results in humans. Studies were also excluded for final evaluation if the agents were not administered on the same day. This exclusion criterion was defined to apply these sequences to how chemotherapy is administered for same-day treatments at an infusion center. If there were conflicting data on a sequence after all studies were evaluated as clinically relevant, recommendations were made to err on the side of safety. In other words, if one study suggested a particular sequence was more toxic and a second study suggested there was no difference, we recommended the sequence that was less toxic rather than stating there was no difference.
Results
The literature search, conducted in October 2009, yielded 110 articles for evaluation. Of those studies, 29 were excluded for testing in vitro sequence only; 11 were excluded as they included only mice; nine were excluded because they did not compare results with the reverse sequence; and four were excluded for other reasons. (Three had trends toward pharmacokinetic changes but did not assess the clinical relevance of these changes, and one used oral agents.) Fifty-seven evaluable articles remained, 44 of which were clinical trials and 13 were literature reviews, typically for a single class of agents.
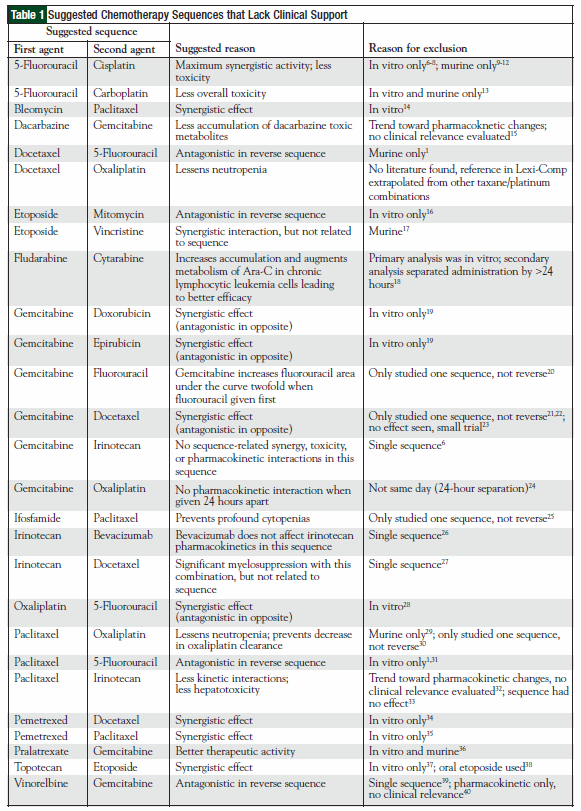
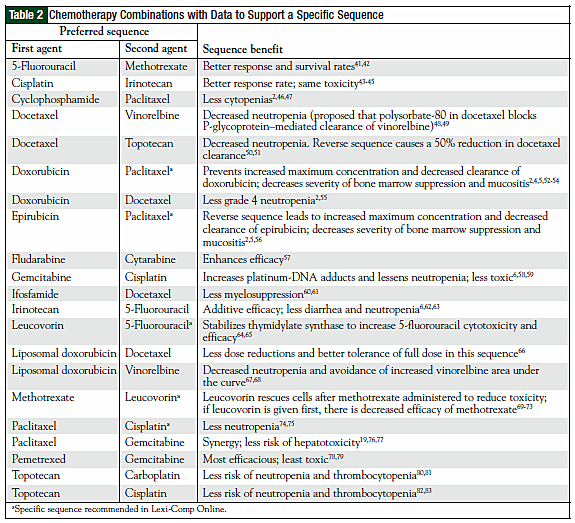
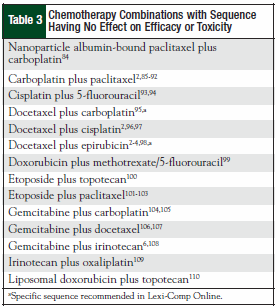
The original 110 articles provided evidence for 62 potential chemotherapy combination sequences. Based on the types of studies examined, 27 potential sequences remained clinically undefined because of a lack of clinical relevance of the supporting studies (Table 1). This left 21 combinations with studies that justified a particular sequence (Table 2), and 14 combinations that provided evidence that sequence was irrelevant to outcomes (Table 3). The combinations with defined sequences or where literature supported no difference in outcomes or toxicity were superimposed onto a compatibility chart for use by our infusion centers (Figure).
Discussion
The goal of this literature search was to create a user-friendly reference chart for pharmacists and nurses who work in outpatient infusion centers and on inpatient oncology floors. We have found that the chart we made has helped quickly define published literature for same-day administration. It must be stated, however, that this chart cannot be used for determining sequences of chemotherapy agents given over several days because that was not the original intent of the literature search.
The literature search revealed that recommendations provided in commonly used drug information resources were based on extrapolated data from drug classes but not studies directly comparing the two agents. An example of this is the taxanes and the platinum agents. We were unable to find any data that directly examine the recommended sequence of docetaxel before oxaliplatin. In addition, the sequence of paclitaxel before carboplatin has been extensively proved to be clinically irrelevant and yet this sequence remains recommended in drug information re sources.2,85-92 These unreferenced recommendations, as one example, could potentially delay treatment, because an oxaliplatin dose could be administered first while docetaxel, which takes longer to reconstitute, is prepared.
We found examples throughout the literature indicating that certain studies may not allow extrapolation to a given sequence. A review by Vaishampayan and colleagues, for instance, demonstrates that in vitro studies cannot be applied in clinical practice. The authors evaluated preclinical and clinical data to show that synergy is not always beneficial.2 When paclitaxel preceded doxorubicin in vitro, they found a threefold increase in cytotoxicity (a synergistic effect); in contrast, when this sequence was studied in humans, they found an increased frequency of mucositis, cardiotoxicity, and neutropenia. Synergistic antitumor activity does not necessarily, however, imply worse clinical toxicity. In a study by Zupi and colleagues, paclitaxel followed by gemcitabine was synergistic in vitro,19 and a study by Poole and colleagues showed that this sequence had less hepatotoxicity and neutropenia than the reverse.76
In studies where a given sequence may have toxicity or efficacy issues, we were unable to conclude that the reverse sequence would resolve the issue. This was demonstrated in a review by Smorenburg and colleagues.1 The authors examined data that suggested the sequence of methotrexate followed by docetaxel was antagonistic and thus had no efficacy, but the reverse sequence caused profound neutropenia, suggesting that this combination of agents should not be used in any sequence. Purely pharmacokinetic studies may not always be applied to their pharmacodynamic properties either. Combination studies of vinorelbine and gemcitabine are an example. In a pharmacokinetic study by Cattel and colleagues, there was an increase in gemcitabine area-under-thecurve and maximum concentration when vinorelbine was administered before gemcitabine.40 The reverse sequence, gemcitabine followed by vinorelbine, was found to be inactive in vivo.39
The comprehensive literature review also revealed several important clinical practice issues that must be evaluated on a consistent basis. One of the common questions encountered by pharmacists in our chemotherapy infusion centers is, “What drug should be administered first?” Pharmacists must use their resources in a timely manner to find the answer—a challenging task given the abundance of literature and resources available. This review has shown that we need solid head-to-head evidence comparing two sequences before we can draw any conclusion. It may not always be the best option to try to extrapolate data from other studies. This includes generalizing data from one agent to the entire therapeutic class, extrapolating data from preclinical studies, or automatically reversing a sequence when the reverse sequence was not directly compared.
Conclusion
As this review has shown, there are several sequences that are well defined in the literature in terms of safety and efficacy. We can use these data, now in a more available format, to help answer some of the common questions regarding chemotherapy administration. At this point, the recommendation for sequence of administration of combination chemotherapy, if not clearly defined in the literature, should follow the sequence of administration published in that regimen’s original study. Further clinical studies will be conducted in the future that continue to elucidate the optimal sequence for all combination agents. We hope to evaluate the efficacy of the chart we devised to see whether it has helped increase efficiency and improve workflow for the pharmacists and nurses at our institution.
Disclosure
Drs Mancini and Modlin did not report any potential financial conflicts of interest.
References
- Smorenburg CH, Sparreboom A, Bontebal M, et al. Combination chemotherapy of the taxanes and antimetabolites: its use and limitations. Eur J Cancer. 2001;37:2310-2323.
- Vaishampayan U, Parchment RE, Jasti BR, et al. Taxanes: an overview of the pharmacokinetics and pharmacodynamics. Urology. 1999;54(6A suppl):22-29.
- Airoldi M, Cattel L, Pedani F, et al. Clinical and pharmacokinetic data of a docetaxel- epirubicin combination in metastatic breast cancer. Breast Cancer Res Treat. 2001;70:185-195.
- Holmes FA, Madden T, Newman RA, et al. Sequence-dependent alteration of doxorubicin pharmacokinetics by paclitaxel in a phase I study of paclitaxel and doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1996;14:2713-2721.
- Danesi R, Conte PF, Del Tacca M. Pharmacokinetic optimisation of treatment schedules for anthracyclines and paclitaxel in patients with cancer. Clin Pharmacokinet. 1999;37:195-211.
- Goel A, Grossbard ML, Malamud S, et al. Pooled efficacy analysis from a phase I-II study of biweekly irinotecan in combination with gemcitabine, 5-fluorouracil, leucovorin and cisplatin in patients with metastatic pancreatic cancer. Anticancer Drugs. 2007;18:263-271.
- Esaki T, Nakano S, Tatsumoto T, et al. Inhibition by 5-fluorouracil of cis-diammine - dichloroplatinum(II)-induced DNA interstrand cross-link removal in a HST-1 human squamous carcinoma cell line. Cancer Res. 1992;52:6501-6506.
- Cho H, Imada T, Oshima T, et al. In-vitro effect of a combination of 5-fluorouracil (5-FU) and cisplatin (CDDP) on human gastric cancer cell lines: timing of cisplatin treatment. Gastric Cancer. 2002;5:43-46.
- Yu NY, Patawaran MB, Chen JY, et al. Influence of treatment sequence on efficacy of fluorouracil and cisplatin intratumoral drug delivery in vivo. Cancer J Sci Am. 1995;1:215-221.
- Kuroki M, Nakano S, Mitsugi K, et al. In vivo comparative therapeutic study of optimal administration of 5-fluorouracil and cisplatin using a newly established HST-1 human squamous-carcinoma cell line. Cancer Chemother Pharmacol. 1992;29:273-276.
- Palmeri S, Trave F, Russello O, et al. The role of drug sequence in therapeutic selectivity of the combination of 5-fluorouracil and cisplatin. Sel Cancer Ther. 1989;5:169-177.
- Pratesi G, Gianni L, Manzotti C, et al. Sequence dependence of the antitumor and toxic effects of 5-fluorouracil and cis-diamminedichloroplatinum combination on primary colon tumors in mice. Cancer Chemother Pharmacol. 1988;21:237-240.
- Saikawa Y, Kubota T, Kuo TH, et al. Combined effect of 5-fluorouracil and carboplatin against human gastric cancer cell lines in vitro and in vivo. Anticancer Res. 1994;14:461-464.
- Waltmire CN, Alberts DS, Dorr RT. Sequence-dependent cytotoxicity of combination chemotherapy using paclitaxel, carboplatin and bleomycin in human lung and ovarian cancer. Anticancer Drugs. 2001;12:595-602.
- Losa R, Fra J, Lopez-Pousa A, et al. Phase II study with the combination of gemcitabine and DTIC in patients with advanced soft tissue sarcomas. Cancer Chemother Pharmacol. 2007;59:251-259.
- Seminara P, Pastore C, Iascone C, et al. Mitomycin C and etoposide in advanced colorectal carcinoma. A clinical and in vitro experience that focuses the problem of schedule dependence in combination therapy. Chemotherapy. 2007;53:218-225.
- Jackson DV Jr, Long TR, Rice DG, et al. Combination vincristine and VP-16- 213: evaluation of drug sequence. Cancer Biochem Biophys. 1986;8:265-275.
- Gandhi V, Kemena A, Keating MJ, et al. Fludarabine infusion potentiates arabinosylcytosine metabolism in lymphocytes of patients with chronic lymphocytic leukemia. Cancer Res. 1992;52:897-903.
- Zupi G, Scarsella M, D’Angelo C, et al. Potentiation of the antitumoral activity of gemcitabine and paclitaxel in combination on human breast cancer cells. Cancer Biol Ther. 2005;4:866-871.
- Correale P, Cerretani D, Marsili S, et al. Gemcitabine increases systemic 5-fluorouracil exposure in advanced cancer patients. Eur J Cancer. 2003;39:1547-1551.
- Dumez H, Louwerens M, Pawinsky A, et al. The impact of drug administration sequence and pharmacokinetic interaction in a phase I study of the combination of docetaxel and gemcitabine in patients with advanced solid tumors. Anticancer Drugs. 2002;13:583-593.
- Leu KM, Ostruszka LJ, Shewach D, et al. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treatment with gemcitabine followed by docetaxel in the treatment of sarcoma. J Clin Oncol. 2004;22:1706-1712.
- Bhargava P, Marshall JL, Fried K, et al. Phase I and pharmacokinetic study of two sequences of gemcitabine and docetaxel administered weekly to patients with advanced cancer. Cancer Chemother Pharmacol. 2001;48:95-103.
- Airoldi M, Cattel L, Passera R, et al. Gemcitabine and oxaliplatin in patients with pancreatic adenocarcinoma: clinical and pharmacokinetic data. Pancreas. 2006;32:44-50.
- Forastiere AA, Urba SG. Single-agent paclitaxel and paclitaxel plus ifosfamide in the treatment of head and neck cancer. Semin Oncol. 1995;22(suppl 6):24-27.
- Denlinger CS, Blanchard R, Xu L, et al. Pharmacokinetic analysis of irinotecan plus bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol. May 5, 2009. Epub ahead of print.
- Adjei AA, Klein CE, Kastrissios H, et al. Phase I and pharmacokinetic study of irinotecan and docetaxel in patients with advanced solid tumors: preliminary evidence of clinical activity. J Clin Oncol. 2000;18:1116-1123.
- Qin B, Tanaka R, Shibata Y, et al. In-vitro schedule-dependent interaction between oxaliplatin and 5-fluorouracil in human gastric cancer cell lines. Anticancer Drugs. 2006;17:445-453.
- Liu J, Kraut EH, Balcerzak S, et al. Dosing sequence-dependent pharmacokinetic interaction of oxaliplatin with paclitaxel in the rat. Cancer Chemother Pharmacol. 2002;50:445-453.
- Liu J, Kraut E, Bender J, et al. Pharmacokinetics of oxaliplatin (NSC 266046) alone and in combination with paclitaxel in cancer patients. Cancer Chemother Pharmacol. 2002;49:367-374.
- Toiyama T, Tanaka K, Konishi N, et al. Administration sequence-dependent antitumor effects of paclitaxel and 5-fluorouracil in the human gastric cancer cell line MKN45. Cancer Chemother Pharmacol. 2006;57:368-375.
- Hotta K, Ueoka H, Kiura K, et al. A phase I study and pharmacokinetics of irinotecan (CPT-11) and paclitaxel in patients with advanced non-small cell lung cancer. Lung Cancer. 2004;45:77-84.
- Murren JR, Peccerillo K, DiStasio SA, et al. Dose escalation and pharmacokinetic study of irinotecan in combination with paclitaxel in patients with advanced cancer. Cancer Chemother Pharmacol. 2000;46:43-50.
- Kano Y, Tanaka M, Akutsu M, et al. Schedule-dependent synergism and antagonism between pemetrexed and docetaxel in human lung cancer cell lines in vitro. Cancer Chemother Pharmacol. 2009;64:1129-1137.
- Kano Y, Akutsu M, Tsunoda S, et al. Schedule-dependent synergism and antagonism between pemetrexed and paclitaxel in human carcinoma cell lines in vitro. Cancer Chemother Pharmacol. 2004;54:505-513.
- Toner LE, Vrhovac R, Smith EA, et al. The schedule-dependent effects of the novel antifolate pralatrexate and gemcitabine are superior to methotrexate and cytarabine in models of human non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:924-932.
- Bonner JA, Kozelsky TF. The significance of the sequence of administration of topotecan and etoposide. Cancer Chemother Pharmacol. 1996;39:109-112.
- Miller AA, Niell HB. Phase I and pharmacologic study of sequential topotecan, carboplatin, and etoposide. Lung Cancer. 2001;33:241-248.
- Juergens R, Brahmer J, Ettinger D. Gemcitabine and vinorelbine in recurrent advanced non-small cell lung cancer: sequence does matter. Cancer Chemother Pharmacol. 2007;59:621-629.
- Cattel L, Airoldi M, Passera R, et al. Gemcitabine plus vinorelbine chemotherapy regimens: a pharmacokinetic study of alternate administration sequences. Pharm World Sci. 2004;26:238-241.
- Mackintosh JF, Coates AS, Tattersall MH, et al. Chemotherapy of advanced head and neck cancer: updated results of a randomized trial of the order of administration of sequential methotrexate and 5-fluorouracil. Med Pediatr Oncol. 1988;16:304-307.
- Coates AS, Tattersall MH, Swanson C, et al. Combination therapy with methotrexate and 5-fluorouracil: a prospective randomized clinical trial of order of administration. J Clin Oncol. 1984;2:756-761.
- Han JY, Lim HS, Lee DH, et al. Randomized phase II study of two opposite administration sequences of irinotecan and cisplatin in patients with advanced non - small cell lung carcinoma. Cancer. 2006;106:873-880.
- de Jonge MJ, Verweij J, de Bruijn P, et al. Pharmacokinetic, metabolic, and pharmacodynamic profiles in a dose-escalating study of irinotecan and cisplatin. J Clin Oncol. 2000;18:195-203.
- de Jonge MJ, Verweij J, Plating AS, et al. Drug-administration sequence does not change pharmacodynamics and kinetics of irinotecan and cisplatin. Clin Cancer Res. 1999;5:2012-2017.
- Kennedy MJ, Zahurak ML, Donehower RC, et al. Sequence-dependent hematologic toxicity associated with the 3-hour paclitaxel/cyclophosphamide doublet. Clin Cancer Res. 1998;4:349-356.
- Kennedy MJ, Zahurak ML, Donehower RC, et al. Phase I and pharmacologic study of sequences of paclitaxel and cyclophosphamide supported by granulocyte colony-stimulating factor in women with previously treated metastatic breast cancer. J Clin Oncol. 1996;14:783-791.
- Airoldi M, Cattell L, Marchionatti S, et al. Docetaxel and vinorelbine in recurrent head and neck cancer: pharmacokinetic and clinical results. Am J Clin Oncol. 2003;26:378-381.
- Airoldi M, Cattel L, Pedani F, et al. Clinical data and pharmacokinetics of a do cetaxel-vinorelbine combination in anthracycline resistant/relapsed metastatic breast cancer. Acta Oncologica. 2003;42:186-194.
- Zamboni WC, Egorin MJ, Van Echo DA, et al. Pharmacokinetic and pharmacodynamic study of the combination of docetaxel and topotecan in patients with solid tumors. J Clin Oncol. 2000;18:3288-3294.
- Posey JA, Wang H, Hamilton J, et al. Phase-I dose escalation and sequencing study of docetaxel and continuous infusion topotecan in patients with advanced malignancies. Cancer Chemother Pharmacol. 2005;56:182-188.
- Frassineti GL, Zoli W, Tienghi A, et al. The sequential administration of combined doxorubicin and paclitaxel in the treatment of advanced breast cancer. Semin Oncol. 1996;23(suppl 12):22-28.
- Gianni L, Munzone E, Capri G, et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol. 1995;13:2688-2699.
- Sledge GW Jr, Robert N, Sparano JA, et al. Eastern Cooperative Oncology Group studies of paclitaxel and doxorubicin in advanced breast cancer. Semin Oncol. 1995;22(suppl 6):105-108.
- Itoh K, Sasak Y, Fujii H, et al. Study of dose escalation and sequence switching of administration of the combination of docetaxel and doxorubicin in advanced breast cancer. Clin Cancer Res. 2000;6:4082-4090.
- Focan C, Graas MP, Beauduin M, et al. Sequential administration of epirubicin and paclitaxel for advanced breast cancer. A phase I randomized trial. Anticancer Res. 2005;25:1211-1217.
- Pastore D, Specchia G, Carluccio P, et al. FLAG-IDA in the treatment of refractory/ relapsed acute myeloid leukemia: single-center experience. Ann Hematol. 2003; 82:231-235.
- Kroep JR, Peters GJ, van Moorsel CJ, et al. Gemcitabine-cisplatin: a schedule finding study. Ann Oncol. 1999;10:1503-1510.
- Van Moorsel CJA, Kroep JR, Pinedo HM, et al. Pharmacokinetic schedule finding study of the combination of gemcitabine and cisplatin in patients with solid tumors. Ann Oncol. 1999;10:444-448.
- Hervonen P, Jekunen A, Lefebvre P, et al. Docetaxel-ifosfamide combination chemotherapy in patients with metastatic hormone-refractory prostate cancer: a phase I pharmacokinetic study. Int J Clin Pharmacol Res. 2003;23:1-7.
- Schrijvers D, Pronk L, Highley M, et al. Pharmacokinetics of ifosfamide are changed by combination with docetaxel: results of a phase I pharmacologic study. Am J Clin Oncol. 2000;23:358-363.
- Mans DR, Grivicich I, Peters GJ, et al. Sequence-dependent growth inhibition and DNA damage formation by the irinotecan-5-fluorouracil combination in human colon carcinoma cell lines. Eur J Cancer. 1999;35:1851-1861.
- Falcone A, Di Paolo A, Masi G, et al. Sequence effect of irinotecan and fluorouracil treatment on pharmacokinetics and toxicity in chemotherapy-naïve metastatic colorectal cancer patients. J Clin Oncol. 2001;19:3456-3462.
- Rustum YM, Cao S, Zhang Z. Rationale for treatment design: biochemical modulation of 5-fluorouracil by leucovorin. Cancer J Sci Am. 1998;4:12-18.
- Jolivet J. Role of leucovorin dosing and administration schedule. Eur J Cancer. 1995;31A:1311-1315.
- Fracasso PM, Rodriguez LC, Herzog TJ, et al. Phase 1 dose and sequencing study of pegylated liposomal doxorubicin and docetaxel in patients with advanced malignancies. Cancer. 2003;98:610-617.
- Cattell L, Passera R, Katsaros D, et al. Pegylated liposomal doxorubicin and vinorelbine in recurrent ovarian carcinoma: a pharmacokinetic study on alternate administration sequences. Anticancer Res. 2006;26:745-750.
- Katsaros D, Oletti MV, Rigault de la Longrais IA, et al. Clinical and pharmacokinetic phase II study of pegylated liposomal doxorubicin and vinorelbine in heavily pretreated recurrent ovarian carcinoma. Ann Oncol. 2005;16:300-306.
- Ravelli A, Migliavacca D, Viola S, et al. Efficacy of folinic acid in reducing methotrexate toxicity in juvenile idiopathic arthritis. Clin Exp Rheumatol. 1999;17:625-627.
- Ortiz Z, Shea B, Suarez-Almazor ME, et al. The efficacy of folic acid and folinic acid in reducing methotrexate gastrointestinal toxicity in rheumatoid arthritis. A metaanalysis of randomized controlled trials. J Rheumatol. 1998;25:36-43.
- Alarcon GS, Morgan SL. Folinic acid to prevent side effects of methotrexate in juvenile rheumatoid arthritis. J Rheumatol. 1996;23:2184-2185.
- Joyce DA, Will RK, Hoffman DM, et al. Exacerbation of rheumatoid arthritis in patients treated with methotrexate after administration of folinic acid. Ann Rheum Dis. 1991;50:913-914.
- Morgan SL, Oster RA, Lee JY, et al. The effect of folic acid and folinic acid supplements on purine metabolism in methotrexate-treated rheumatoid arthritis. Arthritis Rheum. 2004;50:3104-3011.
- Milross CG, Peters LJ, Hunter NR. Sequence-dependent antitumor activity of paclitaxel (Taxol) and cisplatin in vivo. Int J Cancer. 1995;62:599-604.
- Rowinsky EK, Gilbert MR, McGuire WP, et al. Sequences of Taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991;9:1692-1703.
- Poole CJ, Perren T, Gawande S, et al. Optimized sequence of drug administration and schedule leads to improved dose delivery for gemcitabine and paclitaxel in combination: a phase 1 trial in patients with recurrent ovarian cancer. Int J Gynecol Cancer. 2006;16:507-514.
- Oliveras-Ferraros C, Vazquez-Martin A, Colomer R, et al. Sequence-dependent synergism and antagonism between paclitaxel and gemcitabine in breast cancer cells: the importance of scheduling. Int J Oncol. 2008;32:113-120.
- Adjei AA. Clinical studies of pemetrexed and gemcitabine combinations. Ann Oncol. 2006;15(supp 5):v29-v32.
- Ma CX, Nair S, Thomas S, et al. Randomized phase II trial of three schedules of pemetrexed and gemcitabine as front-line therapy for advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5929-5937.
- Simpson AB, Calvert PM, Sludden JA, et al. Topotecan in combination with carboplatin: phase I trial evaluation of two treatment schedules. Ann Oncol. 2002; 13:399-402.
- Boss DS, Siegel-Lakhai WS, van Egmond-Schoemaker NE, et al. Phase 1 pharmacokinetic and pharmacodynamic study of carboplatin and topotecan administered intravenously every 28 days to patients with malignant solid tumors. Clin Cancer Res. 2009;15:4475-4483.
- Rowinsky EK, Kaufmann SH, Baker SD, et al. Sequences of topotecan and cisplatin: phase I, pharmacologic, and in vitro studies to examine sequence dependence. J Clin Oncol. 1996;14:3074-3084.
- Raymond E, Burris HA, Rowinsky EK, et al. Phase I study of daily times five topotecan and single injection of cisplatin in patients with previously untreated nonsmall- cell lung carcinoma. Ann Oncol. 1997;8:1003-1008.
- Stinchcombe TE, Socinski MA, Walko CM, et al. Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors. Cancer Chemother Pharmacol. 2007;60:759-766.
- DiPaola RS, Rubin E, Toppmeyer D, et al. Gemcitabine combined with sequential paclitaxel and carboplatin in patients with urothelial cancers and other advanced malignancies. Med Sci Monit. 2003;9:PI5-PI11.
- Van Warmerdam LJ, Huizing MT, Giaccone G, et al. Clinical pharmacology of carboplatin administered in combination with paclitaxel. Semin Oncol. 1997;24 (suppl 2):97-104.
- Huizing MT, Giaccone G, van Warmerdam LJ, et al. Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer. The European Cancer Centre. J Clin Oncol. 1997;15:317-329.
- Giaccone G, Huizing M, Postmus PE, et al. Dose-finding and sequencing study of paclitaxel and carboplatin in non-small cell lung cancer. Semin Oncol. 1995;22(suppl 9):78-82.
- Perez EA, Hartmann LC. Paclitaxel and carboplatin for advanced breast cancer. Semin Oncol. 1996;23(suppl 11):41-45.
- Obasaju CK, Johnson SW, Rogatko A, et al. Evaluation of carboplatin pharmacokinetics in the absence and presence of paclitaxel. Clin Cancer Res. 1996;2:549-552.
- Belani CP, Kearns CM, Zuhowski EG, et al. Phase I trial, including pharmacokinetic and pharmacodynamic correlations, of combination paclitaxel and carboplatin in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 1999;17:676-684.
- Markman M, Elson P, Kulp B, et al. Carboplatin plus paclitaxel combination chemotherapy: impact of sequence of drug administration on treatment-induced neutropenia. Gynecol Oncol. 2003;91:118-122.
- Koizumi W, Kurihara M, Hasegawa K, et al. Sequence-dependence of cisplatin and 5-fluorouracil in advanced and recurrent gastric cancer. Oncol Rep. 2004; 12:557-561.
- Falcone A, Allegrini G, Masi G, et al. 5-Fluorouracil administered as a 48-hour chronomodulated infusion in combination with leucovorin and cisplatin: a randomized phase II study in metastatic colorectal cancer. Oncology. 2001;61:28-35.
- Ando M, Saka H, Ando Y, et al. Sequence effect of docetaxel and carboplatin on toxicity, tumor response and pharmacokinetics in non-small-cell lung cancer patients: a phase I study of two sequences. Cancer Chemother Pharmacol. 2005; 55:552-558.
- Pronk LC, Schellens JH, Planting AS, et al. Phase I and pharmacologic study of docetaxel and cisplatin in patients with advanced solid tumors. J Clin Oncol. 1997;15:1071-1079.
- Royer I, Monsarrat B, Sonnier M, et al. Metabolism of docetaxel by human cytochromes P450: interactions with paclitaxel and other antineoplastic drugs. Cancer Res. 1996;56:58-65.
- Lunardi G, Venturini M, Vannozzi MO, et al. Influence of alternate sequences of epirubicin and docetaxel on the pharmacokinetic behaviour of both drugs in advanced breast cancer. Ann Oncol. 2002;13:280-285.
- Westermann AM, Taal BG, Swart M, et al. Sequence-dependent toxicity profile in modified FAMTX (fluorouracil-adriamycin-methotrexate) chemotherapy with lenograstim support for advanced gastric cancer: a feasibility study. Pharmacol Res. 2000;42:151-156.
- Huisman C, Postmus PE, Giaccone G, et al. A phase I study of sequential intravenous topotecan and etoposide in lung cancer patients. Ann Oncol. 2001;12:1567-1573.
- Rosell R, Felip E, Massuti B, et al. A sequence-dependent paclitaxel/etoposide phase II trial in patients with non-small cell lung cancer. Semin Oncol. 1997;24(suppl 12):56-60.
- Fleming GF, Roth BJ, Baker SD, et al. Phase I trial of paclitaxel and etoposide for recurrent ovarian carcinoma: a Gynecologic Oncology Group Study. Am J Clin Oncol. 2000;23:609-613.
- Felip E, Massuti B, Camps C, et al. Superiority of sequential versus concurrent administration of paclitaxel with etoposide in advanced non-small cell lung cancer: comparison of two phase II trials. Clin Cancer Res. 1998;4:2723-2728.
- Edelman MJ, Quam H, Mullins B. Interactions of gemcitabine, carboplatin and paclitaxel in molecularly defined non-small-cell lung cancer cell lines. Cancer Chemother Pharmacol. 2001;48:141-144.
- Langer CJ, Calvert P, Ozols RF. Gemcitabine and carboplatin in combination: phase I and phase II studies. Semin Oncol. 1998;25(suppl 9):51-54.
- Harita S, Watanabe Y, Kiura K, et al. Influence of altering administration sequence of docetaxel, gemcitabine and cisplatin in patients with advanced nonsmall cell lung cancer. Anticancer Res. 2006;26:1637-1641.
- Rizvi NA, Spiridonidis CH, Davis TH, et al. Docetaxel and gemcitabine combinations in non-small cell lung cancer. Semin Oncol. 1999;26(suppl 16):27-31, 41-42.
- Ramnath N, Yu J, Khushalani NI, et al. Scheduled administration of low dose irinotecan before gemcitabine in the second line therapy of non-small cell lung cancer: a phase II study. Anticancer Drugs. 2008;19:749-752.
- Gil-Delgado MA, Bastian G, Guinet F, et al. Oxaliplatin plus irinotecan and FU-FOL combination and pharmacokinetic analysis in advanced colorectal cancer patients. Am J Clin Oncol. 2004;27:294-298.
- Dupont J, Aghajanian C, Andrea G, et al. Topotecan and liposomal doxorubicin in recurrent ovarian cancer: is sequence important? Int J Gynecol Cancer. 2006;16(suppl 1):68-73.