Poly (ADP-ribose) polymerase (PARP) inhibitors prevent the repair of single-stranded DNA breaks, leading to double-stranded breaks that cannot be repaired in tumors that are deficient in homologous recombination repair.1 The inability to repair DNA damage causes cell death.1 Currently, there are 4 FDA-approved PARP inhibitors, including olaparib, niraparib, rucaparib, and talazoparib. Olaparib is the only PARP inhibitor approved across 4 oncologic malignancies, including ovarian, breast, pancreatic, and prostate cancers.2-5 In patients with ovarian cancer, PARP inhibitors provide a substantial benefit in progression-free survival compared with placebo after platinum-based chemotherapy, without decreasing quality of life.5-7
Hypersensitivity reactions can be either allergic (immune globulin E [IgE]-mediated, anaphylactic) or nonallergic (non–IgE-mediated, anaphylactoid).8 Anaphylactic reactions are defined as immediate systemic reactions that are caused by a rapid, IgE-mediated immune release of potent mediators from tissue mast cells and peripheral blood basophils.8 Anaphylactoid reactions are immediate systemic reactions that can mimic anaphylaxis but that are not IgE-mediated,8 and instead may be caused by complement activation.9 Hypersensitivity reactions and corresponding desensitization protocols after the use of intravenous chemotherapy agents (eg, platinums, taxanes) and oral drugs such as penicillin are well described in the literature, but are rarely reported with oral oncolytic drugs.10-15
Currently, there are no standard desensitization protocols in the prescribing information for PARP inhibitors, including for olaparib.16 For patients with advanced ovarian cancer and a BRCA1 or BRCA2 mutation who have responded to platinum-based chemotherapy, every effort should be made for the patient to continue receiving a PARP inhibitor.4-7
Case Report
A 70-year-old woman with a medical history significant for breast cancer, hypercholesterolemia, and osteoarthritis presented to her primary care physician with abdominal cramping and pain. She had a left adnexal cyst and extensive pelvic and lower abdominal lymphadenopathies. A core biopsy of a cervical lymph node revealed a poorly differentiated adenocarcinoma of Mullerian origin. She was diagnosed with stage IVB, high-grade adenocarcinoma of Mullerian origin with a germline BRCA1 mutation. After receiving 6 cycles of first-line, guideline-directed, neoadjuvant treatment with carboplatin and paclitaxel, she had a significant decline in CA-125 (cancer antigen 125). Because of the presence of mesenteric disease and inguinal nodes, debulking surgery was not recommended at the end of chemotherapy. The patient was subsequently started on first-line maintenance treatment with olaparib 300 mg twice daily.
Within 2 hours of receiving the first dose of olaparib 300 mg, the patient had facial swelling and a pruritic, painful rash on her face and neck. The rash progressed to areas of her upper trunk, including her back and abdomen. The patient had no other systemic symptoms; however, she presented to urgent care 5 hours after receiving olaparib. Her vital signs at urgent care were stable, including a body temperature of 98.3˚F, pulse of 81 beats per minute, respiratory rate of 18 breaths per minute, and blood pressure of 152/69 mm Hg. Treatment with olaparib was held, and she was seen in the clinic as an urgent care visit by her attending oncologist who prescribed oral diphenhydramine 25 to 50 mg every 6 hours. The patient’s symptoms resolved over the next 48 to 72 hours. She was seen by an allergist/immunologist 10 days later. Although a skin biopsy and skin testing were not completed, based on the onset of her symptoms and their slow resolution over 48 to 72 hours, the allergist felt that the patient’s reaction was consistent with an anaphylactoid non–IgE-mediated hypersensitivity reaction.
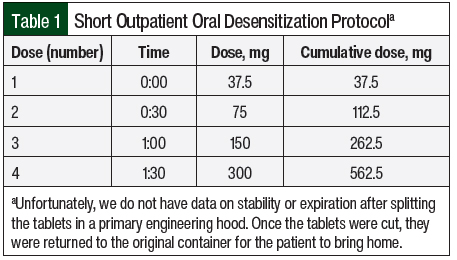
Based on 2 previous case reports of olaparib desensitization, the patient’s allergist proposed a 1-day oral desensitization protocol at his outpatient allergy clinic, which consisted of initiating olaparib at 37.5 mg and then doubling the dose every 30 minutes until reaching 300 mg (Table 1).11,14 Personnel donning personal protective equipment cut olaparib 150-mg tablets into quarters inside of a containment primary engineering control to produce 37.5-mg tablets. The patient subsequently commenced the oral desensitization protocol at the allergist’s clinic under close surveillance by medical staff. Approximately 30 minutes after the last dose of the protocol (300 mg), the patient again had a rash, which was not accompanied by systemic symptoms concerning for an IgE-mediated anaphylactic reaction. She received oral medications, including prednisone 60 mg, cetirizine 10 mg, and diphenhydramine 50 mg, and was observed for 3 hours before being discharged home from the clinic.
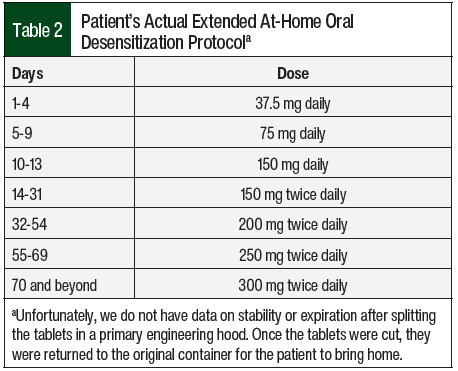
In collaboration with her allergist, we weighed the benefits and risks of attempting a second desensitization protocol. We proposed an extended at-home oral desensitization protocol, in which the patient was to receive cetirizine 10 mg before each dose of olaparib. Olaparib was planned to be initiated at 37.5 mg, continued daily for 4 days, and then doubled every 4 days to reach 300 mg twice daily in 16 days. The patient was also prescribed oral diphenhydramine 25 to 50 mg and triamcinolone cream as needed.
The patient preferred a slower increase than what was originally planned. Over 10 weeks, she received cetirizine 10 mg before each dose of olaparib and slowly doubled the dose and/or frequency of olaparib to reach the full dose of 300 mg twice daily (Table 2). She tolerated all doses throughout the entirety of the 10-week desensitization period with no recurrence of hypersensitivity reaction; currently, 8 months later, she is stable and receiving treatment with olaparib.
Discussion
We describe the first case of a successful extended at-home olaparib desensitization protocol. To our knowledge, only 3 case reports of olaparib desensitization have been published, and they were completed over the course of 1 to 2 days in a clinic setting under close observation by medical staff.11,14,17 No case reports of extended at-home olaparib desensitization with minimal monitoring have been published to our knowledge. In addition, to our knowledge, no desensitization protocols for other oral oncolytics—including for PARP inhibitors, such as niraparib, rucaparib, and talazoparib—have been published.
Grabowski and colleagues describe a successful 2-day olaparib desensitization protocol in a clinic setting of a 49-year-old woman with platinum-sensitive relapse of a high-grade serous peritoneal cancer.11 After receiving platinum-based chemotherapy, the patient was initiated on olaparib 400 mg twice daily, but she had an allergic reaction after the first dose. Her allergic reaction was characterized by angioedema and cutaneous wheals, which happened reproducibly within 3 hours after each olaparib dose and lasted for several hours. Three attempts to rechallenge with premedications were unsuccessful. Skin-prick testing confirmed IgE-mediated hypersensitivity reaction to olaparib. As a result, a 2-day desensitization protocol was developed with oral administration of incremental doses of olaparib, starting at 12.5 mg and ending at 800 mg. No additional medications were used throughout the desensitization, and no adverse events were observed. At the end of the first day of treatment, the patient had mild, transient urticaria. No hypersensitivity symptoms were noted on day 2. In the 16-month period after the desensitization, the patient continued receiving olaparib 400 mg twice daily with no further hypersensitivity.
Zhu and colleagues describe a successful 1-day olaparib desensitization in a clinic setting of a 58-year-old woman with recurrent high-grade serous ovarian cancer who had cytoreductive surgery and received intraperitoneal chemotherapy and carboplatin plus paclitaxel and was initiated on treatment with olaparib 300 mg twice daily.14 Approximately 35 minutes after receiving the first dose of olaparib, the patient had extreme facial flushing and diffuse pruritus. Immediately after receiving the second dose of olaparib, she had lip and facial angioedema and pruritus. After the third dose of olaparib, she had diffuse pruritus, facial urticaria, periorbital and lip angioedema, and nausea within 30 minutes. She discontinued treatment with olaparib, and her symptoms resolved within 24 hours after receiving oral diphenhydramine. The patient was admitted to the inpatient service and successfully underwent a 9-step, 1-day desensitization program. She received oral cetirizine 20 mg, oral montelukast 10 mg, intravenous ranitidine 50 mg, and intravenous dexamethasone 10 mg as premedications before the desensitization. Although the patient had several isolated erythematous macules during the desensitization, she did not have urticaria, angioedema, or systemic side effects. She was able to continue receiving olaparib with no interruptions or interventions in treatment.
Hypersensitivity reactions have been reported in <20% of patients receiving rucaparib, in 2% of patients receiving olaparib, and in an uncertain number of patients receiving niraparib in the postmarketing experience; hypersensitivity reactions have not been reported in patients receiving talazoparib.16,18-20 The exact mechanism of hypersensitivity reactions to PARP inhibitors remains unclear.
Our patient did not tolerate the initial short 1-day olaparib desensitization protocol performed in the allergist’s clinic. A possible reason for this could be that she received a cumulative dose of olaparib of 562.5 mg in the span of 90 minutes. Although a phase 1 study previously demonstrated the tolerability of olaparib 600 mg twice daily, the maximum tolerated dose was deemed to be 400 mg twice daily (capsule formulation) after the study had concluded.21 We initially chose this protocol because we hoped to ensure that our patient would be able to tolerate a single 300-mg dose of olaparib at home. Because the 150-mg tablets of olaparib could only be cut into quarters (four 37.5-mg sections), in increasing in a stepwise fashion to 300 mg per dose the total cumulative dose was 562.5 mg. This finding illustrates the potential importance of proceeding with treatment at low doses and increasing the dose slowly over time, as in the extended at-home protocol.
Our case of a successful extended at-home desensitization protocol for olaparib is unique because it is the first report of oral oncolytic desensitization completed at home without intensive monitoring by medical staff. This case could help to inform future patients who have hypersensitivity reactions to oral oncolytics. A patient’s oncology and allergy care team should carefully weigh the risks and benefits of pursuing an at-home desensitization protocol for oral oncolytics. Consideration must be given to several factors, including the patient’s severity of previous symptoms, propensity to report a reaction, social support, proximity to an emergency department, and cancer-specific characteristics.
Conclusion
Our patient’s case demonstrates the first successful at-home olaparib desensitization protocol in a woman with advanced cancer of Mullerian origin with germline BRCA1 mutation. Her symptoms after the first dose of olaparib suggested a non–IgE-mediated anaphylactoid reaction. She did not tolerate the previously published 1-day olaparib desensitization protocols, so we created our own extended at-home olaparib desensitization protocol, which the patient tolerated well. She continues to receive olaparib today without the recurrence of hypersensitivity reaction.
This case demonstrates that patients who have hypersensitivity reactions may be slowly desensitized at home safely. Patients who have hypersensitivity reactions to PARP inhibitors should be considered for an extended desensitization protocol such as ours to allow them to realize the benefits of PARP inhibitor maintenance.
Author Disclosure Statement
Dr Shea was a consultant on an advisory board to GlaxoSmithKline; Dr Jia, Dr Sendrowski, and Dr Lax have no conflicts of interest to report.
References
- McFarlane T, Rehman N, Wang K, et al. Cutaneous toxicities of new targeted cancer therapies: must know for diagnosis, management, and patient-proxy empowerment. Ann Palliat Med. 2020;9:1296-1306.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091-2102.
- Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523-533. Erratum in: N Engl J Med. 2017;377:1700.
- Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317-327.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- González-Martín A, Pothuri B, Vergote I, et al; for the PRIMA/ENGOT-OV26/GOG-3012 investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75-87.
- American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology. The diagnosis and management of anaphylaxis: VI. Definitions of anaphylaxis and anaphylactoid events. J Allergy Clin Immunol. 1998;101(6):S481.
- Lagopoulous V, Gigi E. Anaphylactic and anaphylactoid reactions during the perioperative period. Hippokratia. 2011;15:138-140.
- Sullivan TJ, Yecies LD, Shatz GS, et al. Desensitization of patients allergic to penicillin using orally administered β-lactam antibiotics. J Allergy Clin Immunol. 1982;69:275-282.
- Grabowski JP, Sehouli J, Glajzer J, et al. Olaparib desensitization in a patient with recurrent peritoneal cancer. N Engl J Med. 2018;379:2176-2177. Erratum in: N Engl J Med. 2019;380:1386.
- Lee CW, Matulonis UA, Castells MC. Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol. 2004;95:370-376.
- Fishman A, Gold T, Goldberg A, et al. Effective desensitization protocol to paclitaxel following hypersensitivity reaction. Int J Gynecol Cancer. 1999;9:156-159.
- Zhu R, Welch S, Roberts H. Successful olaparib desensitization using a novel one-day protocol. Allergy Asthma Clin Immunol. 2020;16:100.
- Wendel GD, Stark BJ, Jamison RB, et al. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med. 1985;312:1229-1232.
- Lynparza (olaparib) tablets, for oral use [prescribing information]. AstraZeneca Pharmaceuticals; October 2020.
- Beurer BM, Sprenger LM, Graneß K, et al. Novel approach of desensitization in allergic reaction to olaparib. J Oncol Pharm Pract. Published online September 21, 2022. doi:10.1177/10781552221124041.
- Talzenna (talazoparib) tablets, for oral use [prescribing information]. Pfizer Laboratories; September 2021.
- Rubraca (rucaparib) tablets, for oral use [prescribing information]. Clovis Oncology; December 2022.
- Zejula (niraparib) capsules, for oral use [prescribing information]. GlaxoSmithKline; April 2023.
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123-134.