Immune checkpoint inhibitors are monoclonal antibodies that are designed to upregulate the body’s immune system through T-cell activation.1,2 These immunotherapies work to prevent malignant cells from evading apoptosis through 3 main mechanisms, including inhibition of programmed cell death 1 (PD-1), PD ligand 1 (PD-L1), and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4).1,2 Currently, 7 immune checkpoint inhibitors (pembrolizumab, nivolumab, cemiplimab, atezolizumab, avelumab, durvalumab, and ipilimumab) are available and are indicated for various malignancies.
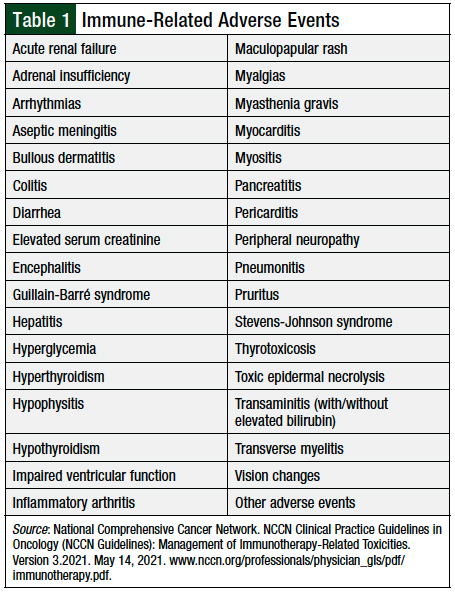
Although immune checkpoint blockade has added to the available treatment options for patients with cancer, they carry a unique risk for immune-related adverse events (irAEs). These irAEs can manifest in many organ systems. The most common irAEs include rash, pruritus, colitis, hepatitis, pneumonitis, hypo- or hyperthyroidism, hypophysitis, and adrenal insufficiency (Table 1).
These adverse events vary in severity and may lead to treatment delay or discontinuation. One meta-analysis described an incidence of grade ≥3 irAEs between approximately 7% and 55%.3 The rate of irAEs was dependent on the checkpoint inhibitor class, and whether patients received checkpoint inhibitors as monotherapy or as part of a combination regimen (ie, nivolumab plus ipilimumab). As can be expected, combination therapy conferred higher rates of irAEs compared with monotherapy.3
It is difficult to predict which patients will have irAEs, and when they may occur. A postulated risk factor for irAEs is the concurrent use of vaccines and immune checkpoint blockade. T-cells are exhausted by overexposure to antigens, and the inhibition of PD-1, PD-L1, or CTLA-4 restores their function, creating a hyperactive state for T-cells.4,5 New antigen exposure from inactivated vaccines, which consist of modified viruses, bacteria, or pathogens that are unable to replicate once administered, allows for T-cell interactions that may drive increased irAEs.4,5
Current guidelines from the Infectious Diseases Society of America and the National Comprehensive Cancer Network (NCCN) recommend that patients with active cancer receive the inactivated or recombinant influenza vaccine annually.6,7 The NCCN’s guidelines on the management of irAEs further specify that inactivated or killed vaccines are permissible in patients who are receiving immunotherapy.8
Other inactivated vaccinations, including pneumococcal and meningococcal vaccines, are also recommended for specific patients.6,7 The NCCN guidelines recommend against using live vaccines in patients with cancer, because of a lack of clear information.7,8
Literature describing the incidence and severity of irAEs with the use of inactivated vaccines is limited and only describes the risks associated with influenza vaccination. In 2018, one study showed that patients receiving PD-1 inhibitor therapy with nivolumab or with pembrolizumab concurrent with inactivated influenza vaccines had a higher incidence of grade 3 or 4 irAEs than historical controls.9
More recent studies, however, contradicted these data and did not show an increased incidence of irAEs.4,10 Wijn and colleagues demonstrated that in patients who received nivolumab, the rates of treatment discontinuation were similar between the patients who received and those who did not receive the influenza vaccine.10
Despite growth in the study of immunotherapies, few primary studies evaluated inactivated vaccines other than influenza vaccines concurrently with immunotherapy. Furthermore, there are limited published data regarding the use of vaccines in patients who are receiving immunotherapy beyond nivolumab or pembrolizumab, or in combination with chemotherapy.4,9-11 It is pertinent to address the safety of inactivated vaccines when used with immune checkpoint inhibitor therapy.
At the University of Kansas Health System, various inactivated vaccines have been administered to patients who are receiving immune checkpoint inhibitor therapy. Currently, no institutional policies are in place for the use of inactivated vaccines in patients who are receiving immunotherapy, because individual providers determine vaccine use. This study analyzed the incidence of irAEs in patients receiving a broad scope of inactivated vaccines and immune checkpoint inhibitor therapies at the University of Kansas Health System.
Methods
This retrospective, single-center chart review included a cohort of patients aged ≥18 years who received an inactivated vaccine 30 days before or 60 days after the administration of immune checkpoint inhibitor therapy between January 1, 2015, and July 31, 2019. Patients were excluded if they had a documented allergy or allergic reaction to an inactivated vaccine, received immunosuppressive therapy for organ transplant or autoimmune disease, had a history of irAE requiring a high-dose corticosteroid (ie, prednisone ≥20 mg or an equivalent dose) before vaccination, or received an investigational immunotherapy regimen.
The vaccinated cohort was matched based on age and immune checkpoint inhibitor in a 1:2 ratio to control patients who had no documentation of vaccine administration within 30 days before or 60 days after receiving an immune checkpoint inhibitor.
This study was approved by the Institutional Review Board of the University of Kansas Medical Center. The primary objective was the relative incidence rate of irAEs requiring intervention in the vaccinated group versus in the control group. The secondary objectives were the rates of immune checkpoint inhibitor therapy delay or discontinuation because of irAEs.
A therapeutic intervention for an irAE was defined as a delay of immune checkpoint inhibitor treatment by ≥14 days after the expected date of therapy for cycle 2 or beyond, discontinuation of treatment, or the addition of supportive therapy.
Supportive therapy was defined as the addition of an immunosuppressive medication for the management of irAEs, such as corticosteroids, immunosuppressants (ie, mycophenolate, infliximab, azathioprine, cyclosporine, cyclophosphamide, rituximab, tocilizumab, methotrexate, leflunomide, anti-thymocyte globulin), sulfasalazine, or intravenous immunoglobulin. Only irAEs that newly occurred after receipt of a vaccine and an immune checkpoint inhibitor were included in the study.
The data collected included patient baseline demographics, malignancy type, vaccine type and administration date, immune checkpoint inhibitor regimen and dates of administration, hospitalizations, and supportive therapy use. The reasons for therapy delays and discontinuation, hospitalization, and supportive medication were evaluated to determine if they were the result of an irAE. Outcome data were evaluated with an independent chi-square test.
Results
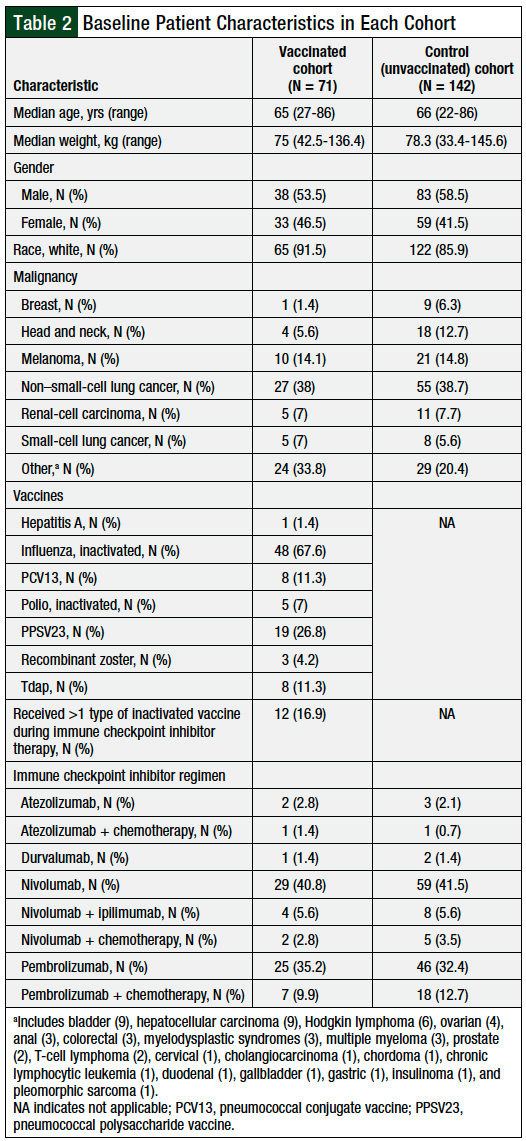
A total of 71 patients met the study inclusion criteria for the vaccinated cohort. These patients were matched against 142 patients in the control group, bringing the total number of patients to 213. The baseline demographics were similar between the groups (Table 2). The patients’ most common malignancies included non–small-cell lung cancer, melanoma, renal-cell carcinoma, small-cell lung cancer, and head and neck cancer.
The inactivated influenza vaccine was the most frequently (N = 48) administered, followed by pneumococcal vaccines (PPSV23, N = 19; PCV13, N = 8) and the Tdap vaccine (N = 8). Other vaccines included inactivated polio (N = 5), recombinant zoster (N = 3), and hepatitis A (N = 1). In addition, 12 (16.9%) patients received more than 1 type of inactivated vaccine while receiving immunotherapy (Table 2).
Patients in the vaccinated cohort most often received nivolumab monotherapy (40.8%) or pembrolizumab monotherapy (35.2%); fewer patients received nivolumab plus ipilimumab (5.6%), nivolumab plus chemotherapy (2.8%), or pembrolizumab plus chemotherapy (9.9%). The other immune checkpoint inhibitor therapies used in the vaccinated cohort were atezolizumab monotherapy, atezolizumab plus chemotherapy, and durvalumab (Table 2).
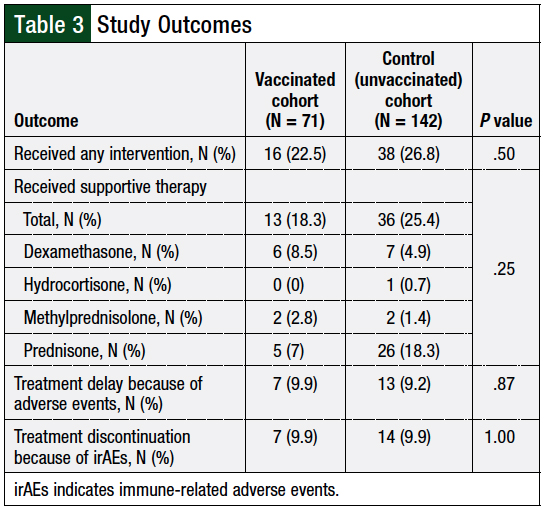
A total of 22.5% (16) of vaccinated patients had an irAE requiring an intervention versus 26.8% (38) of patients in the control cohort (P = .50; Table 3). Furthermore, no significant differences were observed in the rates of therapy delays resulting from irAEs (vaccinated 9.9% vs control 9.2%; P = .87) or discontinuations resulting from toxicity (vaccinated 9.9% vs control 9.9%; P = 1.00; Table 3). Other reasons for therapy delays in the study population included schedule changes, hospitalizations for reasons not related to irAEs, and surgery or radiation.
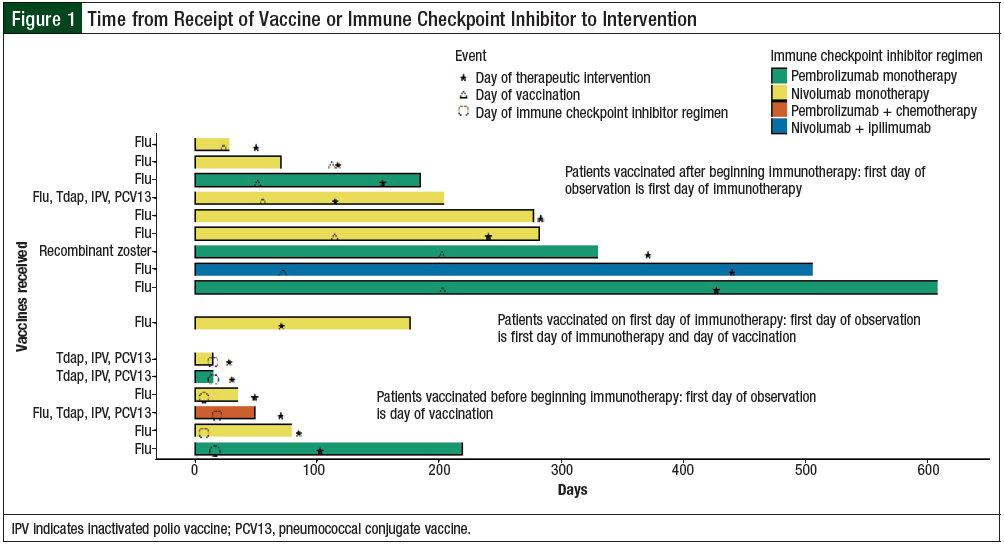
The swimmer plot depicted in Figure 1 highlights patients who were vaccinated and required a therapeutic intervention. The duration of time receiving immune checkpoint inhibitor therapy is shown relative to vaccine and immune checkpoint inhibitor administration, as well as when patients required an intervention. Multiple patients continued to receive checkpoint inhibitor therapy beyond the time of an intervention. In contrast, some patients did not require an intervention until after the checkpoint inhibitor therapy ended. The time between vaccine administration and the intervention is also variable and does not follow a clear pattern.
For the patients who started checkpoint inhibitor therapy before vaccination, the median time from vaccination to intervention was 60 days (range, 0-368 days). In patients who were vaccinated first, the median time from initiation of immune checkpoint inhibitor therapy to intervention was 74.5 days (range, 14-86 days). For the 1 patient who received a vaccine on the same day that immune checkpoint inhibitor therapy was initiated, the time to intervention was 71 days.
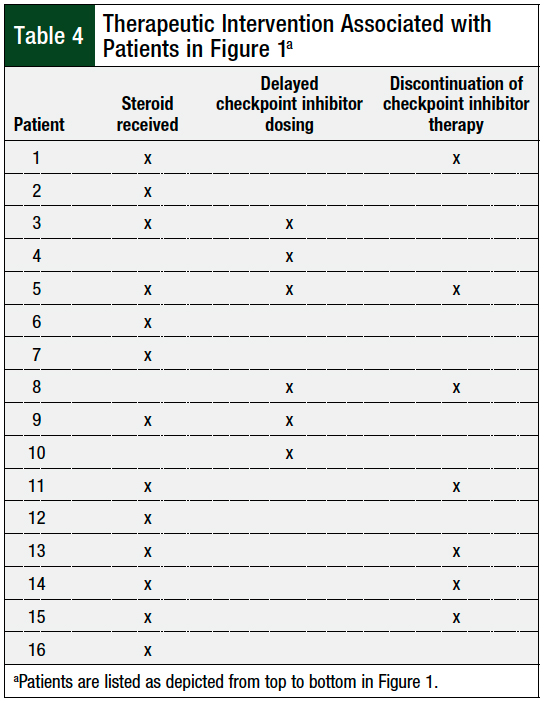
Table 4 includes every patient shown in Figure 1, and whether that patient’s interventions included corticosteroids, treatment delay, treatment discontinuation, or a combination of those modalities.
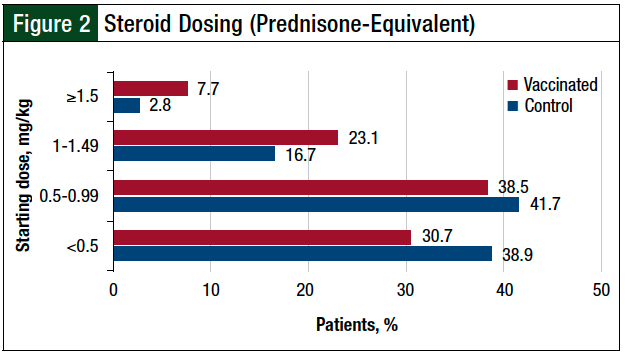
The reasons for immunotherapy discontinuation other than irAEs included death, disease progression, therapy completion, patient preference, changes in therapy, and transfer of care to other institutions. All patients requiring the use of supportive therapy received a corticosteroid, and no difference was seen in the rate of patients requiring additional therapy between the 2 cohorts (vaccinated, 18.3%; control, 25.4%; P = .25; Table 3). Most patients received a starting steroid dose of <1 mg/kg of prednisone (or prednisone equivalent), as shown in Figure 2.
No patients received a different immunosuppressant for an irAE. A total of 20 (9.4%) patients across both cohorts received a corticosteroid for an alternative indication other than an irAE during the study period. These indications varied and included (but were not limited to) brain metastases, pain, and fatigue. Of these patients, only 2 (both in the vaccinated cohort) required an intervention, one of whom received dexamethasone 8 mg daily for brain metastases. This patient subsequently had pneumonitis, requiring methylprednisolone 500 mg daily and immune checkpoint inhibitor discontinuation. The other patient discontinued treatment as the sole intervention.
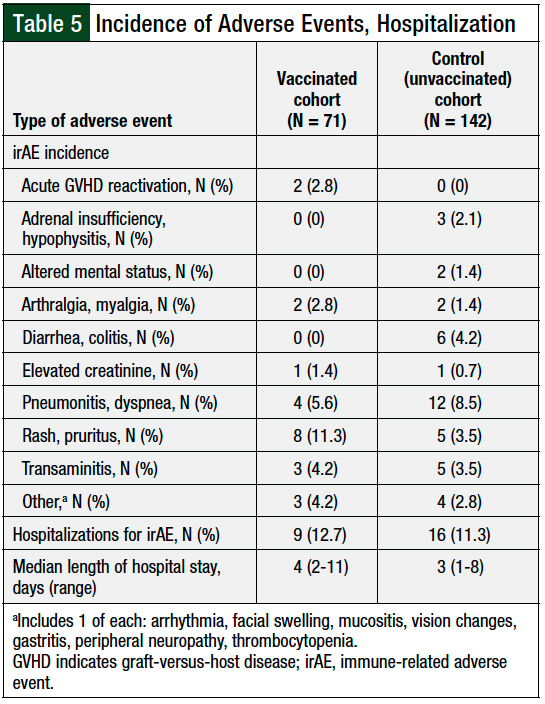
The common irAEs requiring an intervention included rash or pruritus, pneumonitis or dyspnea, diarrhea or colitis, and transaminitis (Table 5).
Patients infrequently required hospitalization for irAEs, and the incidence of irAE-related hospitalization was similar between the cohorts: 12.7% of vaccinated patients versus 11.3% of the unvaccinated patients (P = .76; Table 5).
The patients who received vaccines were hospitalized for a median of 4 days (range, 2-11 days) versus a median of 3 days (range, 1-8 days) for patients who were not vaccinated.
Discussion
The immune system upregulation driven by immune checkpoint inhibitor therapy frequently causes adverse effects that, in some cases, dramatically affect patients and their care. Although in many cases these effects may be managed with short breaks in therapy, patients often require the addition of immunosuppressive medications, significant delays in treatment, and the discontinuation of therapy altogether.
The incidence of irAEs in conjunction with inactivated influenza vaccination is an ongoing clinical controversy and studies assessing other vaccinations are lacking. Expanding the scope of understanding how various inactivated vaccines and immunotherapy work in concert to cause irAEs will give clinicians an increased knowledge needed to engage in informed medical decision-making. The increased receipt of appropriate vaccines would provide optimized care to an already vulnerable patient population.
Compared with a matched control cohort, the 71 patients who received checkpoint inhibitor therapy and concurrent vaccination in our study did not increase the incidence of interventions. The patients in the 2 cohorts required a similar rate of treatment delays or discontinuations related to an irAE. The rate of intervention in the vaccinated patients was measured as a surrogate marker for the incidence of irAEs.
Although our study included grade 3 or 4 irAEs that required significant intervention, the results of our study are similar to historical data from a meta-analysis by El Osta and colleagues.3 This study showed an incidence of grade 3 or 4 irAEs between 6.3% and 21.5%, depending on the medication used. For combination immunotherapy, the incidence of grade 3 or 4 irAEs was nearly 55%.3
All patients in our study who required the addition of supportive therapy were given corticosteroids, although the initial dosing varied widely and highlights differences in provider practices. The majority of patients received a prednisone or prednisone-equivalent dose of <1 mg/kg (Figure 2). This dosing may indicate that although patients required supportive therapy, providers were not characterizing every irAE as a severe grade. It also highlights the spectrum and complexity of the patients’ clinical presentation.
We did not evaluate patient outcomes based on differences in steroid dosing. Because the study defined the receipt of high-dose corticosteroids as an intervention, the patients receiving over-the-counter topical steroids for a dermatologic reaction were assumed to have a less severe irAE and thus were not captured in the study.
It is notable that no patients required nonsteroidal immunosuppressive therapy, such as infliximab. We therefore presumed that none of the patients’ irAEs were refractory to their management (ie, treatment delay, treatment discontinuation, or receiving a corticosteroid).
A noteworthy irAE in 2 patients who received vaccines was graft-versus-host disease (GVHD) reactivation after the initiation of immune checkpoint inhibitor therapy, which followed allogeneic stem-cell transplant. A total of 4 patients in the vaccinated cohort had a history of allogeneic stem-cell transplant. These patients were not excluded, to facilitate a wholistic and real-world perspective. The high incidence of GVHD in this setting has been described in previous studies.12,13
Of these 2 patients, the first had Hodgkin lymphoma and began nivolumab therapy on day 266 post-transplant, 2 weeks after receiving the inactivated vaccines. The patient subsequently had a rash, transaminitis, and presumed acute GVHD, which the transplant team attributed to receiving nivolumab.
The patient received corticosteroids and discontinued immune checkpoint inhibitor therapy after only 1 dose. The patient’s liver biopsy showed intraepithelial lymphocytosis, bile duct damage, and pronounced cholestasis consistent with acute GVHD.
The second patient had peripheral T-cell lymphoma and began treatment with pembrolizumab on day 488 post-transplant, which was 2 weeks after receiving inactivated vaccines. After the first dose, the patient had indeterminate immune-related pneumonitis or acute GVHD, and pembrolizumab treatment was discontinued, because pembrolizumab could not be excluded as the causative agent.
In both cases, the patients’ complex clinical scenario made it difficult to correlate definitively acute GVHD with immune checkpoint inhibitor therapy, but the medications may be contributing factors, given the acute changes in clinical condition and proximity to receipt of immune checkpoint inhibitor therapy.
The risk surrounding the coadministration of vaccines and immune checkpoint inhibitors is an important consideration. In this study, we used a more stringent time frame than previous studies, by limiting the receipt of a vaccine to only 30 days before an immune checkpoint inhibitor administration.4 We allowed for vaccination up to 60 days after immune checkpoint inhibitor administration, given the long half-life of these medications.
Chong and colleagues included any patients receiving an inactivated influenza vaccine and an immune checkpoint inhibitor within 65 days, showing that the probability of having an irAE over time was not influenced by the order in which the vaccine and the immune checkpoint inhibitor were given.4 These authors also found no difference in the incidence of irAEs if an influenza vaccine was administered the same date as an immune checkpoint inhibitor.4
This was also allowed and assessed in our study, where only 1 patient who received an inactivated vaccine the same day as the first dose of immune checkpoint inhibitor therapy had an irAE requiring intervention (Figure 1).
Chong and colleagues also noted that the risk factors for irAEs included older age and the receipt of combination immune checkpoint inhibitor therapy.4 These risk factors were controlled in our study through the cohort-matching methodology. Patients were matched based on age, and those who received combination immune checkpoint inhibitor therapy were further matched to help prevent confounding.
Several studies have evaluated influenza vaccines in patients receiving immune checkpoint inhibitor therapy and show similar risk for adverse events.11,14 The study by Gwynn and colleagues specifically demonstrated similarities in serum cytokine and chemokine levels.11
To our knowledge, our study is one of the first studies to include data of inactivated vaccines beyond influenza and with regimens combining traditional chemotherapy and checkpoint inhibitor therapy. The results in our study are congruent with the 2017 study by Schenk that showed that routine vaccination (ie, influenza and pneumococcal) in patients receiving checkpoint inhibitor therapy did not increase the number or severity of adverse events.15 This is the only other study that included data on a vaccine other than influenza.15
A study by Läubli and colleagues has demonstrated similar seroprotection rates when administering influenza vaccines to patients receiving immune checkpoint inhibitor therapy compared with healthy controls.9 The incidence of similar seroprotection rates remains to be explored with other inactivated vaccines, such as the vaccines reported in our study. Because of the limited sample size, further evaluation of patients receiving traditional chemotherapy with immune checkpoint inhibitor therapy is needed to characterize safety in this patient population.
As providers become increasingly comfortable with vaccinating patients who are receiving checkpoint inhibitor therapy, and as more regimens combine immune checkpoint inhibitor therapy with traditional cytotoxic chemotherapy, the results of our study should help to increase providers’ confidence with administering the full scope of guideline-recommended vaccines for their patients who are receiving immune checkpoint inhibitor therapy.
The risk for patients who are not vaccinated may significantly outweigh the risk for irAEs. Future studies could include measuring the incidence of vaccine-preventable infections and its impact on patients’ morbidity and mortality. The pharmacoeconomic impact of patients who are not receiving appropriate vaccinations should also be considered.
Limitations
This study has several limitations. The nature of retrospective chart review is a limitation, because of the reliance on providers’ clinical progress notes within the electronic medical record system. Adverse event grading and reporting could be inconsistent or incomplete. For this reason, grading scales were not used to classify irAEs in this study.
Another limitation is the use of the surrogate marker for the primary end point. Specifically, patients who did not require supportive immunosuppressive therapy or a 14-day treatment delay or discontinuation, but were managed with shorter therapy delays or other supportive care, would not have met the primary end point of our study. Therefore, the cumulative incidence of irAEs is likely higher than what was presented here.
Another limitation is the use of the electronic medical records system to determine the patients in the control cohort. Despite the ability to pull in immunization records from outside facilities, there is still a chance that a patient was vaccinated at another facility. Those records could theoretically not have been reconciled and not included in our health system registries; however, we believe that the chance of this happening is minimal. This limitation is also likely representative of other health systems, and this problem would be true for other analyses.
In addition, patients received different types of immunotherapy regimens, so comparison of adverse events by type of vaccination did not seem appropriate.
Finally, patients meeting the inclusion criteria near the end of the study period likely did not have their full therapy assessed and might have had an irAE after the study period. Because the study time frame was the same for the 2 cohorts, patients from either cohort might have had additional irAEs that could not be assessed; therefore, the effect of this remains unclear.
Conclusion
In this study, immune checkpoint inhibitor treatment delays and discontinuations were minimal and similar between patients who were vaccinated and those who were not, indicating that patients who are receiving such treatment should not expect to have their duration of therapy influenced by the administration of an inactivated vaccine. Patients also required a limited number of hospitalizations because of irAEs, and those hospitalized had a relatively short length of stay, suggesting that patients are not expected to require frequent or prolonged hospitalizations. Patients who are receiving immune checkpoint inhibitor therapy may continue to receive inactivated vaccines without an increased risk for irAEs that require an intervention. Providers should therefore continue to recommend inactivated vaccines to the appropriate patients.
To our knowledge, this is one of few studies that provides information on the use of inactivated vaccines beyond influenza. Our findings confirm that vaccination is a safe, guideline-recommended practice.
Author Disclosure Statement
Dr Kurkowski, Dr Ralph, and Dr Grauer have no conflicts of interest to report. Dr Martin is an Advisory Board consultant to G1 Therapeutics and to Clovis Oncology. Dr Mahmoudjafari has participated in the Advisory Board of Omeros, Genentech, Legend Biotech, and Celgene. Dr Wulff-Burchfield has provided consulting to Astellas and to Exelixis, and is on the Advisory Board of Exelixis.
References
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86.
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
- El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1-12.
- Chong CR, Park VJ, Cohen B, et al. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70:193-199.
- Finnefrock AC, Tang A, Li F, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980-987.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44-e100. Erratum in: Clin Infect Dis. 2014;59:144.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prevention and Treatment of Cancer-Related Infections. Version 1.2021. www.nccn.org/professionals/physician_gls/pdf/infections.pdf. Accessed July 16, 2021.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Management of Immunotherapy-Related Toxicities. Version 3.2021. May 14, 2021. www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed July 16, 2021.
- Läubli H, Balmelli C, Kaufmann L, et al. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J Immunother Cancer. 2018;6:40.
- Wijn DH, Groeneveld GH, Vollaard AM, et al. Influenza vaccination in patients with lung cancer receiving anti–programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur J Cancer. 2018;104:182-187.
- Gwynn ME, DeRemer DL, Saunders KM, et al. Immune-mediated adverse events following influenza vaccine in cancer patients receiving immune checkpoint inhibitors. J Oncol Pharm Pract. 2020;26:647-654.
- Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017;129:2471-2478.
- Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post–allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130:221-228.
- Failing JJ, Ho TP, Yadav S, et al. Safety of influenza vaccine in patients with cancer receiving pembrolizumab. JCO Oncol Pract. 2020;16:e573-e580.
- Schenk EL. Clinical outcomes of patients on check point inhibitor therapy who receive routine vaccinations. J Clin Oncol. 2017;35(15_suppl):Abstract e14597.