In the United States, the most frequently diagnosed central nervous system (CNS) tumors are brain metastases.1 Approximately 20% to 40% of patients with systemic cancer will have CNS metastasis during the course of their disease.2 Currently, lung cancer, breast cancer, and melanoma are the prominent causes of brain metastases.1,3
The treatment options for brain metastases include surgical resection, whole-brain radiation therapy, and stereotactic radiosurgery, or any combination of these 3 options.3 Stereotactic radiosurgery is a highly precise radiation therapy that was initially developed for the treatment of small brain tumors and functional abnormalities of the brain.3 Stereotactic radiosurgery leads to brain necrosis in approximately 10% of patients.4
Brain radionecrosis is an inflammatory reaction that can occur between 6 and 24 months after stereotactic radiosurgery.3,5 Radiation brain necrosis is characterized by an increased permeability of capillaries and a disruption of the blood–brain barrier.3,5 This disruption results in the leakage of capillaries leading to increased oxygen-free radicals, a proinflammatory milieu, and the increased production of vascular endothelial growth factor (VEGF).6
The incidence of brain radionecrosis depends on the dose of radiation, fractionation, amount of chemotherapy exposure, location of tumor, and patient-specific factors.3,6 The incidence of brain radionecrosis is between 5% and 25%.7,8
Differentiating between brain radionecrosis and tumor progression can be difficult, and pathologic diagnosis via biopsy is seen as the gold standard.9 The process can be invasive and challenging because of the location of the disease.9 Therefore, less-invasive means, such as a comprehensive imaging modality, are used to diagnose brain radionecrosis, including medical history, signs and symptoms, magnetic resonance imaging (MRI), and positron emission tomography (PET)/computed tomography scans.10,11
Patients with brain metastases can have a medley of symptoms, including headaches, seizures, cognitive impairment, fatigue, nausea, and focal deficits.1,6 Focal deficits include problems with nerve, spinal cord, or brain function.12 Issues in these areas may lead to speech, vision, and hearing problems as well.12
The treatment of brain radionecrosis ranges from the use of conservative methods in patients who are asymptomatic to the use of medical management, hyperbaric oxygen therapy, and surgical interventions.6,13 Historically, the mainstay of treatment for brain radionecrosis has been high-dose corticosteroids.6,13 Due to the significant adverse events associated with the use of corticosteroids,6,13 however, there is a need for alternative treatment options. Because there is an increased production of VEGF with brain radionecrosis, studies have evaluated the use of bevacizumab as a potential treatment option for brain radionecrosis.13-15
Bevacizumab is an anti-VEGF antibody.16 Through the inhibition of microvascular growth, bevacizumab stops the growth of tissue cells.16 Elevated VEGF levels have been found in several incidences of brain necrosis.10 The presence of VEGF may perpetuate the condition and lead to further blood–brain barrier dysfunction and brain edema.13,15 Therefore, using an agent such as bevacizumab to block VEGF from reaching its capillary targets can be considered a viable treatment option for patients with radiation necrosis.17
Gonzalez and colleagues conducted a retrospective study of 8 patients with primary brain cancer who received bevacizumab for the treatment of brain radionecrosis that was diagnosed by MRI.18 The patients received bevacizumab or bevacizumab in combination with other agents in a dosage of 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks. Investigators noted 48% and 60% decreases in maximum bidirectional measurements for postcontrast T1 and fluid-attenuated inversion recovery (FLAIR) images, respectively, after a mean of 8.1 weeks from the start of bevacizumab treatment.18
A systematic review conducted by Delishaj and colleagues included 21 studies and 125 cases in which bevacizumab had been used for the treatment of brain radionecrosis.14 This review showed that bevacizumab was most frequently dosed at 7.5 mg/kg for 2 weeks for a median of 4 cycles. The results showed a clinical benefit with the improvement of neurologic symptoms in 114 (91.2%) patients. Overall, 122 (97.6%) had radiographic improvement after treatment with bevacizumab, and 3 (2.4%) patients had radiographic progression at the first follow-up.14
In 2021, a systematic review by Liao and colleagues reported the efficacy and safety of bevacizumab.19 In all, 8 retrospective, 2 prospective, and 2 randomized controlled trials (RCTs) were included in this review, with 236 patients with radiation brain necrosis who received bevacizumab. The results showed that 127 (91%) of the patients across 7 studies had clinical improvement. In the 2 RCTs, significant improvement was seen in radiographic responses in the patients who received bevacizumab versus the patients who received corticosteroids or placebo.19 All of the studies showed a reduction on T1 gadolinium enhancement MRI and/or T2 FLAIR MRI images.19 The results showed that treatment with bevacizumab can improve clinical outcomes and cognitive function and may be more efficacious than corticosteroid-based treatment.19
A prospective, randomized study by Levin and colleagues evaluated bevacizumab’s effect on radiographic and clinical activity in 14 patients with primary brain tumors and brain radionecrosis.20 The study showed average percentage change decreases in brain radionecrosis volume on postcontrast T1 and FLAIR studies of –59% and –63%, respectively. All patients who received bevacizumab had an improvement in neurologic symptoms or signs.20
Boothe and colleagues conducted a retrospective review of patients who received bevacizumab for the treatment of brain radionecrosis secondary to stereotactic radiosurgery.5 The study included 11 patients, of whom 6 had metastatic non–small-cell lung cancer and 5 had metastatic breast cancer. The results from this study confirm that treatment with bevacizumab can result in a dramatic decrease in postcontrast T1 enhancement and edema.5
A literature search revealed that there is a paucity of data on the clinical benefit of using bevacizumab for the treatment of brain radionecrosis. Further studies are needed to establish diagnostic and treatment regimens for bevacizumab. This study helped to evaluate the use of bevacizumab for brain radionecrosis therapy secondary to the treatment of brain metastases.
Because the data are limited regarding the use of bevacizumab for the treatment of brain necrosis, it is imperative that more studies are conducted to determine its clinical benefit. Our study focused on patients who are receiving bevacizumab for the treatment of brain radionecrosis to determine the standard doses used, the type of radiation therapy used, and the clinical outcome for each encounter.
The aim of our study was to assess the effectiveness and safety of bevacizumab in the treatment of brain radionecrosis.
Methods
This study was a retrospective chart review of patients who received bevacizumab at Memorial Cancer Institute, in Hollywood, FL, between January 1, 2014, and November 30, 2019. The Memorial Cancer Institute’s database was used to identify the patients who received bevacizumab for the treatment of brain radionecrosis.
Patients were included if they were aged ≥18 years and had brain necrosis that was treated with bevacizumab. Patients were excluded if they had brain necrosis and did not receive bevacizumab treatment or if bevacizumab was used for a different indication.
The diagnosis of brain radionecrosis was determined using the radiologists’ clinical notes in the patients’ charts. Brain imaging, mainly MRIs and PET scans, was completed before treatment and after 4 cycles of therapy to evaluate the efficacy of bevacizumab.
The primary study end point was to determine if the patients who received bevacizumab for the treatment of brain radionecrosis showed a clinical benefit, which was defined as a complete response, partial response, or stable disease on MRI as evaluated by a radiologist. The clinical benefit included MRI findings and the presence of clinical symptoms. Patients who demonstrated a clinical benefit received an additional 4 cycles of treatment with bevacizumab.
The secondary outcome was to assess the safety of bevacizumab for the treatment of brain radionecrosis in adults by evaluating the treatment-related adverse events. The clinical symptoms related to brain radionecrosis were identified and were documented during the study period.
Descriptive statistics were collected to identify the patients’ baseline characteristics. The baseline characteristics and additional data that were analyzed included diagnosis, age, type of radiation therapy received, cancer diagnosis, clinical benefit achieved, dose and duration of bevacizumab treatment, toxicities, and adverse events. Descriptive statistics were used to evaluate the patients’ baseline characteristics.
Results
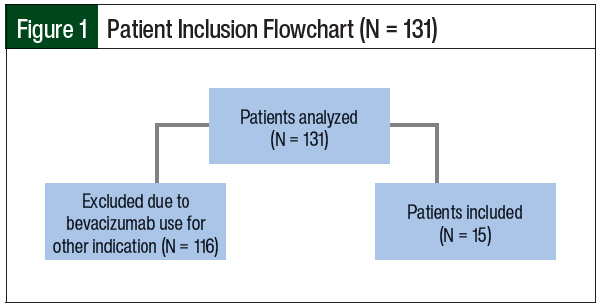
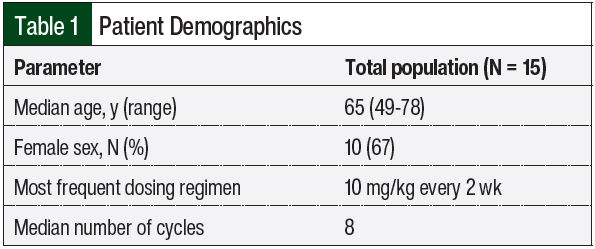
A total of 131 patients were reviewed between January 1, 2014, and November 30, 2019, and 15 patients were included in the final analysis (Figure 1). Table 1 shows the main demographics of the patients enrolled in the study and the dosing intervention that was used. The median patient age was 65 years (range, 49-78 years), and most patients were women (67%).
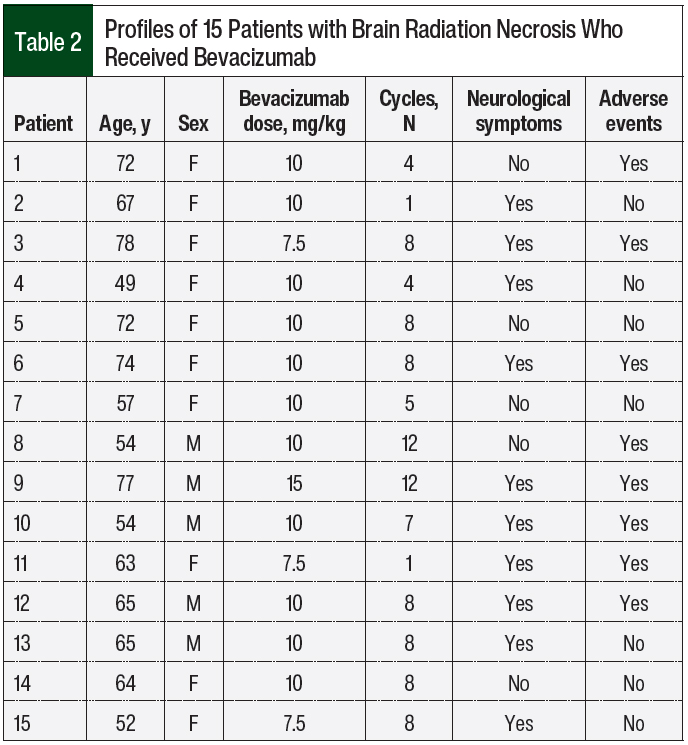
Table 2 indicates whether the patients had neurological symptoms and details the bevacizumab dosing. The most frequent dosing regimen of bevacizumab was 10 mg/kg every 2 weeks, and the median number of cycles given was 8 (range, 1-12 cycles).
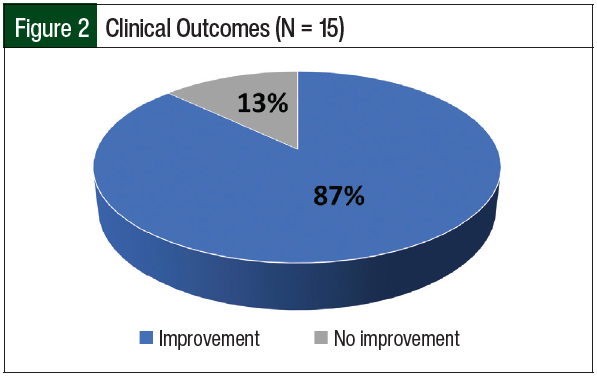
Figure 2 shows that 13 (87%) of the 15 patients had a clinical benefit from treatment with bevacizumab, which was shown by MRI. The patients also had improvements in cognitive function, headaches, weakness, and other symptoms. MRIs demonstrated an improvement in edema and a reduction in or stabilization of brain lesions.
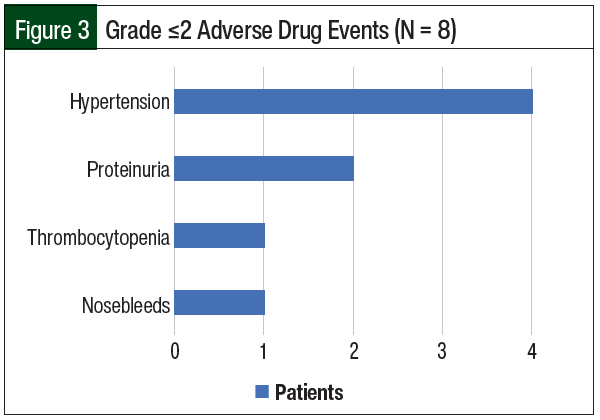
The patients’ adverse events are shown in Figure 3. Treatment with bevacizumab was well-tolerated, with 8 (53%) patients having grade ≤2 bevacizumab-related adverse events, including hypertension (27%), proteinuria (13%), thrombocytopenia (7%), and mild nosebleeds (7%). No grade 3 to 5 adverse events were noted.
Discussion
This study evaluated the effectiveness and safety of bevacizumab for the treatment of brain radionecrosis. Our results are similar to those of previous studies, which show that the majority of the patients who received bevacizumab had an overall clinical benefit.5,6,17,18,20 In addition, most patients had primary lung carcinoma of origin before receiving radiation therapy, which can account for the prevalence of brain metastases from lung cancer.
Up to 65% of patients with lung cancer may have brain metastases.21 Previous studies have not determined an optimal dose or administration schedule for bevacizumab in this patient population.
The most common dose of bevacizumab used in this subset of patients was 10 mg/kg every 2 weeks, which is consistent with the results of 5 mg/kg to 10 mg/kg in the scarce literature that is available.11,14,15 Our study showed clinical benefit in patients who received 4 cycles of bevacizumab and an additional 4 cycles after imaging and had improvement in or no progression of brain radionecrosis. Our study revealed that only 2 patients had worsening of brain radionecrosis after they received treatment with bevacizumab.
The patients in the study tolerated the bevacizumab dosing regimen relatively well. Common side effects of treatment with bevacizumab included epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, and hemorrhage. The study’s median age of 65 years suggests that this dosing regimen may be tolerated well in an older patient population.
Limitations
This study has several limitations. First, the small sample size is a major study limitation.
In addition, this was a retrospective chart analysis. All data were collected by retrospectively reviewing the physicians’ notes to evaluate each patient.
Clinical benefit was based on whether the imaging results showed stable disease, progressive disease, or improvement of disease. The previous treatment regimens that each patient received were not included for analysis to determine if the previous therapy may have an impact on patient outcomes.
Most patients in this study were diagnosed with lung cancer, and only 1 patient was diagnosed with breast cancer. The results may vary in patients with different tumor types.
Although this is a small series of patient cases, further studies are warranted to establish the role of bevacizumab in the treatment of radiation brain necrosis.
Conclusion
The results of this study are consistent with the limited studies that are available in the literature. A new protocol was implemented in our institute to have a default treatment dosage for bevacizumab of 10 mg/kg every 2 weeks for 8 cycles. Patients will receive 4 cycles of bevacizumab followed by brain imaging and then an additional 4 cycles of bevacizumab if they achieve clinical benefit.
Because there is no standard of care for the treatment of brain radionecrosis, more prospective RCTs are needed to assess the benefit of bevacizumab as therapy for brain radionecrosis.
Funding Source
Funding for this study was provided by Memorial Healthcare System.
Author Disclosure Statement
Dr Brahim is Medical Science Liaison of Janssen Pharmaceuticals; Dr Raez has received research support from Genentech and Roche; Dr Below, Dr Khan, and Ms Izquierdo have no conflicts of interest to report.
References
- Ostrom QT, Huang Wright C, Barnholtz-Sloan JS. Brain metastases: epidemiology. In: Schiff D, Van den Bent MJ, eds. Metastatic Disease of the Nervous System. Aminoff MJ, Boller F, Swaab DF, series eds. Handbook of Clinical Neurology. Vol 149 (3rd series). Amsterdam, Netherlands: Elsevier; 2018:27-42.
- Villano JL, Durbin EB, Normandeau C, et al. Incidence of brain metastasis at initial presentation of lung cancer. Neurooncol. 2015;17:122-128.
- Franchino F, Rudà R, Soffietti R. Mechanisms and therapy for cancer metastasis to the brain. Front Oncol. 2018;8:161. doi: 10.3389/fonc.2018.00161.
- Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9:180-188.
- Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neurooncol. 2013;15:1257-1263.
- Vellayappan B, Tan CL, Yong C, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018;8:395. doi: 10.3389/fonc.2018.00395.
- Rahmathulla G, Recinos PF, Valerio JE, et al. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90:192-200.
- Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125:149-156.
- Furuse M, Nonoguchi N, Yamada K, et al. Radiological diagnosis of brain radiation necrosis after cranial irradiation for brain tumor: a systematic review. Radiat Oncol. 2019;14:28. doi: 10.1186/s13014-019-1228-x.
- Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87:449-457.
- Zhuang H, Yuan X, Chang JY, et al. Exploration of the recurrence in radiation brain necrosis after bevacizumab discontinuation. Oncotarget. 2016;7:48842-48849.
- Wippold FJ; Expert Panel on Neurologic Imaging. Focal neurologic deficit. AJNR Am J Neuroradiol. 2008;29:1998-2000.
- Ali FS, Arevalo O, Zorofchian S, et al. Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. 2019;21:66. doi: 10.1007/s11912-019-0818-y.
- Delishaj D, Ursino S, Pasqualetti F, et al. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9:273-280.
- Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18:21. doi: 10.1186/s12943-019-0950-1.
- Avastin (bevacizumab) injection, for intravenous use [prescribing information]. Genentech; September 2022. www.gene.com/download/pdf/avastin_prescribing.pdf. Accessed March 13, 2023.
- Loganadane G, Dhermain F, Louvel G, et al. Brain radiation necrosis: current management with a focus on non-small cell lung cancer patients. Front Oncol. 2018;8:336. doi: 10.3389/fonc.2018.00336.
- Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323-326.
- Liao G, Khan M, Zhao Z, et al. Bevacizumab treatment of radiation-induced brain necrosis: a systematic review. Front Oncol. 2021;11:593449. doi: 10.3389/fonc.2021.593449.
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487-1495. Erratum in: Int J Radiat Oncol Biol Phys. 2012;84:6.
- Chi A, Komaki R. Treatment of brain metastasis from lung cancer. Cancers (Basel). 2010;2:2100-2137.