Ovarian cancer is the leading cause of death among gynecologic tumors.1 First-line management for ovarian cancer consists of surgery plus platinum-based combination chemotherapy, typically cisplatin or carboplatin, with the addition of a taxane, either paclitaxel or docetaxel.2 Nevertheless, more than 70% of patients experience relapse after first-line therapy.3 Second-line treatment depends on a variety of factors, the most important of which is the duration of initial remission, defined as the time between the end of the last treatment course and the diagnosis of disease progression.
For platinum-sensitive disease (ie, platinum-free interval of 6 months or more), treatment of recurrent disease is a platinum-based therapy with the option of adding gemcitabine, bevacizumab, or pegylated liposomal doxorubicin (PLD). For platinum-resistant disease (ie, platinum-free interval of less than 6 months), second-line options most often include topotecan, PLD, paclitaxel, bevacizumab, oral etoposide, and gemcitabine.2
PLD is a widely used agent in the treatment of patients with platinum-sensitive or platinum-resistant recurrent or refractory ovarian cancer.1,4 PLD consists of doxorubicin hydrochloride encased in a 100-nm unilamellar liposome with polyethylene glycol–linked phosphoethanolamine lipid. Compared with conventional doxorubicin, the liposomal formulation significantly increases circulation time in the blood from less than 1 hour for free doxorubicin to approximately 55 hours with the liposomal formulation and has increased cytotoxic effects caused by the enhanced permeability and retention characteristics of tumor tissues.5 In addition, the liposomal formulation reduces the significant cardiac toxicity often associated with conventional doxorubicin.6
Approved by the US Food and Drug Administration (FDA) in 1995, Doxil was the first PLD marketed in the United States. Starting in late 2011, the drug manufacturer had various manufacturing and regulatory hurdles leading to a shortage of Doxil, as well as of other drugs produced in their supplier’s Bedford, OH, plant.7 In response to recurrent shortages of Doxil, the FDA temporarily approved the importation of another PLD, Lipodox from India, as an alternative to Doxil.
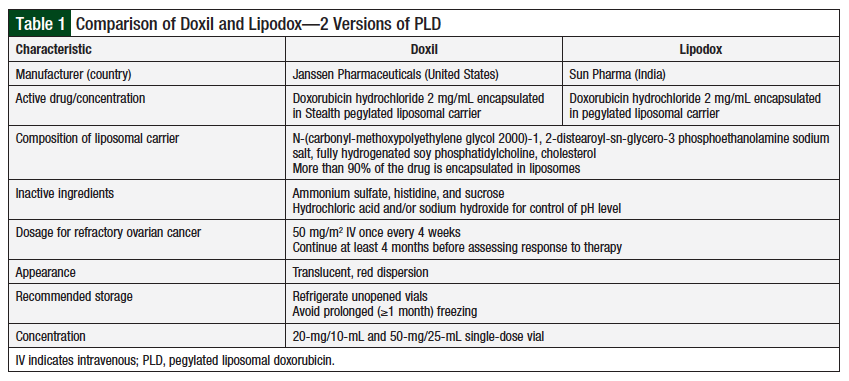
Although the 2 drugs have very similar composition and characteristics (Table 1),8 clinical data comparing their equivalence with regard to efficacy and toxicity are limited.9,10 Two US clinical trials suggested that Doxil and Lipodox may have different clinical activity, resulting in different patient outcomes.9,10
In our institution, we have treated patients using Doxil and/or Lipodox based on drug availability. Because of the nuanced nature of the liposomal drugs and the limited data concerning the therapeutic equivalency of these 2 agents, we compared the treatment outcomes of patients with ovarian cancer who received treatment with Lipodox versus those who received treatment with Doxil.
The objectives of the study were to compare the clinical outcomes of patients who received treatment with 2 different formulations of PLD for recurrent or refractory ovarian cancer, and to assess the impact of the Doxil supply shortage and the use of Lipodox on patient outcomes.
Methods
This study was a retrospective, single-center chart review approved by the Institutional Review Board. Consent procedures were deemed unnecessary, because this was a retrospective review, with no patient contact.
Patients were identified using electronic pharmacy dispensing records and if they received either Lipodox or Doxil. The chart abstraction dates were between January 1, 2007, and July 30, 2014. This date range was chosen to obtain comparable numbers of patients in the Doxil group and the Lipodox group based on the period before and after the Doxil shortage.
Patient charts were deidentified and were reviewed to gather pertinent information using a data collection form. Platinum-free interval was defined as the interval from the last date of platinum-based therapy until documentation of progressive disease.
Study inclusion criteria were patients aged >18 years who received treatment at our institution with a PLD between January 1, 2007, and July 30, 2014, and a diagnosis of recurrent or refractory ovarian cancer. Patients were excluded if they received a PLD for disease other than ovarian cancer. In addition, patients who received combination treatment with a PLD (eg, carboplatin plus a PLD) were excluded from this study, to eliminate those not receiving a PLD as a confounding variable (our study analyzed PLD as monotherapy). Overall, 16 patients were included in the Doxil group, and 20 patients in the Lipodox group; in addition, 4 patients who received both PLD drugs were analyzed as a separate group.
The study’s outcome measures included objective response rate (ORR) and time to progression (TTP). ORRs were based on the Response Evaluation Criteria in Solid Tumors (RECIST).11 TTP was defined as an objective tumor progression evidenced by imaging results or by symptomatic disease worsening based on the physician’s clinical judgment.
Statistical analyses were performed by the institution’s Biostatistics Shared Resource (BIOS Core). Wilcoxon P values were used to analyze patient age, body mass index, number of comorbidities, number of previous chemotherapy regimens, and platinum-free interval. Fisher P values were used to analyze patient race, specific comorbidities, whether the patient had previously received PLD, the ORR, as well as the overall response rate. Kaplan-Meier analysis with log-rank testing was used to assess TTP, with multivariable Cox regression used to control for differences in patient populations. We were not able to perform a power analysis, because there are scant high-quality data on estimates of treatment effect differences (ie, TTP and ORR) between Doxil and Lipodox. All analyses were performed using SAS Statistical Software.
Results
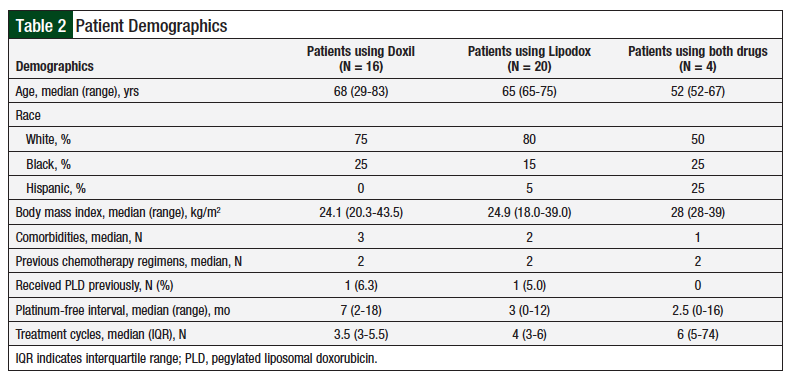
The 2 patient populations had similar demographics in terms of age, race, body mass index, and the number of previous chemotherapy regimens (Table 2). Patients who received Doxil had more comorbidities than patients who received Lipodox (P = .05), which was considered not significant. Patients who received Doxil had a longer platinum-free interval (P = .02), suggesting that their disease was initially more sensitive to platinum-based chemotherapy regimens.
The median number of PLD cycles in the Doxil group and the Lipodox group was 3.5 and 4, respectively, a difference that was not significant (P = .23). In both the Doxil and the Lipodox groups, 1 patient had previously received PLD treatment.
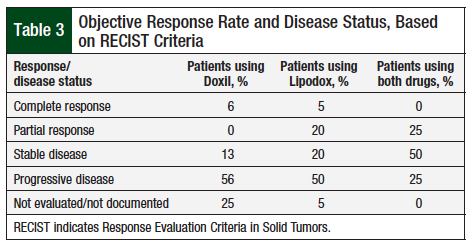
In comparing the ORR using RECIST criteria (Table 3), no significant differences were observed between Doxil and Lipodox (all group comparisons, Fisher test P = .19). In addition, when comparing overall response rate (complete response and partial response), the difference between Doxil and Lipodox was not significant (6% vs 25%; P = .36).
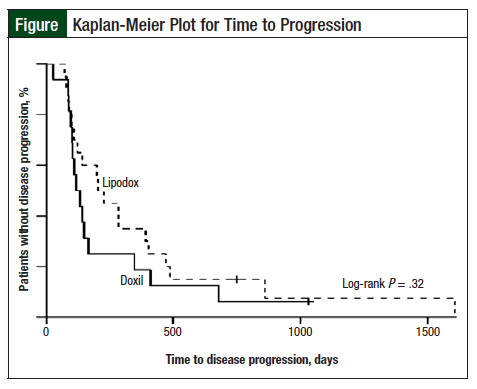
The Lipodox group had a median TTP of 213 days (95% confidence interval [CI], 98-404) and the Doxil group had a median TTP of 123 days (95% CI, 95-164). Although this difference in TTP appears large, a log-rank test did not show a significant difference, with P = .32 (Figure). In addition, after controlling for comorbidities and for platinum-free interval, no significant difference was found in the TTP based on the drug used (hazard ratio [HR], 0.70; P = .31 vs HR, 0.86; P = .68, Lipodox vs Doxil, respectively).
Discussion
Temporary importation of drugs not approved by the FDA is a strategy the FDA turns to during domestic drug shortages. Often, these unapproved drugs have been studied only in patient populations different from those in the United States. For example, Lipodox clinical data are much more robust in Japanese and Taiwanese populations.12,13 For drugs that have been shown to be effective and safe in foreign populations, clinicians and regulatory agencies must consider whether the same efficacy and safety can be extrapolated to patients living in the United States, who may have different environmental exposures and different cancer biology.
The results of our study suggest that there were no differences in therapeutic outcomes in patients with recurrent or refractory ovarian cancer who received Lipodox compared with those who received Doxil. Although patients who received Lipodox had a higher overall response rate and a longer TTP, the difference was not significant, even after controlling for differences in the patient population.
After controlling for the greater number of comorbidities in the Doxil group, the Lipodox group had an HR of 0.70 (P = .31). After controlling for the difference in platinum-free interval between the 2 groups, the Lipodox group had an HR of 0.86 (P = .68).
These data demonstrate no significant difference in ORR or TTP in patients who received Lipodox compared with those who received Doxil, suggesting that the Doxil drug shortage did not adversely affect treatment outcomes when Lipodox was used as a monotherapy for recurrent or refractory ovarian cancer.
Our findings are different from those found by Berger and colleagues in their 2014 comparison of outcomes in 18 patients with recurrent epithelial ovarian cancer who received Lipodox compared with historical Doxil controls.10 The authors found that in their small population of patients who received a median of 3 previous chemotherapy regimens, Lipodox did not produce any complete or partial response as measured by RECIST.10
One reason for this conflict is that the patient population in their study was more heavily pretreated than those in our study, with a median of 3 previous chemotherapy regimens10 compared with a median of 2 regimens in our study. This indicates that ovarian cancer may be more resistant to pharmacologic treatment. In addition, their study included only 18 patients,10 compared with 40 patients in our study.
Limitations
Several limitations in our study provide avenues for future research. First, because of several factors, only a small patient population was available for analysis in our chart review. Despite the date range spanning 7 years, only 40 patients met the study inclusion criteria.
In addition, we were unable to perform a power analysis, because of the scarcity of data concerning differences in TTP and ORR between patients with ovarian cancer who used the 2 formulations of PLD. Without estimates in treatment effect differences, a power analysis could not be performed; it is possible that we were unable to detect a difference between the 2 PLD formulations because of the lack of power.
Furthermore, our study investigated PLD only as a monotherapy, but PLD is often used in combination with other agents in the setting of relapsed or refractory ovarian cancer. This is most relevant with platinum-sensitive relapsed disease, when PLD is often used in conjunction with carboplatin. In platinum-resistant disease, bevacizumab is frequently used in combination with PLD. One potential area of future research is examining outcomes when different PLD formulations are used in combination with other agents.
Another reason for the small patient population was the recurrent drug supply shortage. PLD was difficult to obtain over an extended period, and it is likely that physicians opted for alternate therapies for patients with relapsed disease.
Our study did not capture dose density of the PLD used in the Doxil and Lipodox groups. Whereas the number of cycles did not differ significantly between the 2 groups, our results could have been influenced by the doses of PLD. PLD 50 mg/m2 every 4 weeks is the dose approved for ovarian cancer, but this is often reduced to 40 mg/m2 every 4 weeks to avoid dose-related adverse events in patients who may be heavily pretreated.14 Future research into this topic could benefit from including analysis of dose density.
Finally, this was a single-center study. It is possible that the results of this study could be used as a basis for a larger comparison of outcomes of patients who receive Doxil versus Lipodox, with an analysis powered to detect a potential difference between Doxil and Lipodox. Other hospitals in our healthcare system are now using the same electronic medical record system, so future research could include these hospitals’ patients, increasing the sample size and the power of the analysis to detect a true difference that may exist.
Conclusion
Potential direction for future research is further examination of patients who received both Doxil and Lipodox. The 2014 US study showed that patients with ovarian cancer who received both Doxil and Lipodox had a longer TTP and higher overall response rate compared with patients who received only 1 formulation of PLD.9 In our study, only 4 patients received both drugs, which was too small a sample to analyze. If more patients are analyzed in future research, it would be interesting to see if there is benefit in receiving both Doxil and Lipodox. One potential mechanism of this therapeutic benefit is small differences in liposomal formulation resulting in alterations in how doxorubicin is unloaded from liposomes within tumor cells. This could lead to increased drug exposure at the tumor site when both Doxil and Lipodox are administered.
This retrospective chart review is the largest study to date that directly compares patient outcomes with Lipodox therapy versus Doxil therapy in patients with recurrent or refractory ovarian cancer. The results suggest that the PLD drug shortage of Doxil did not result in differences in therapeutic outcomes in patients who received Lipodox compared with those who received Doxil as a monotherapy for recurrent or refractory ovarian cancer. These data support the use of either drug in the event of a future PLD shortage.
Acknowledgment
The authors acknowledge Allison M. Deal, MS, of University of North Carolina’s Lineberger Comprehensive Cancer Center Biostatistics Shared Resource, for her expert assistance with the statistical analysis.
Author Disclosure Statement
Dr Amerine and Dr Gardiner have no conflicts of interest to report. Dr Shen was a pharmacy student during the study and is now an employee of United Therapeutics Corporation.
References
- Staropoli N, Ciliberto D, Botta C, et al. Pegylated liposomal doxorubicin in the management of ovarian cancer: a systematic review and metaanalysis of randomized trials. Cancer Biol Ther. 2014;15:707-720.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines (NCCN Guidelines): Ovarian Cancer, Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1.2016. June 3, 2016. www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed January 21, 2017.
- Ozols RF, Bundy BN, Greer BE, et al; for the Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194-3200.
- Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312-3322.
- Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419-436.
- Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (Doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029-1033.
- Loftus P. New lapses at J&J’s Doxil supplier. The Wall Street Journal. December 9, 2011. www.wsj.com/articles/SB10001424052970203413304577086432685952686. Accessed October 4, 2016.
- American Society for Health-System Pharmacists. Comparison of Doxil and Lipodox. www.ashp.org/DocLibrary/Policy/DrugShortages/Doxil-Comparison-Table.pdf. March 2012.
- Smith JA, Costales AB, Jaffari M, et al. Is it equivalent? Evaluation of the clinical activity of single agent Lipodox compared to single agent Doxil in the ovarian cancer treatment. J Oncol Pharm Pract. 2016;22:599-604.
- Berger JL, Smith A, Zorn KK, et al. Outcomes analysis of an alternative formulation of PEGylated liposomal doxorubicin in recurrent epithelial ovarian carcinoma during the drug shortage era. Onco Targets Ther. 2014;7:1409-1413. Erratum in: Onco Targets Ther. 2015;8:593.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Chou HH, Wang KL, Chen CA, et al; for the Taiwanese Gynecologic Oncology Group. Pegylated liposomal doxorubicin (Lipo-Dox) for platinum-resistant or refractory epithelial ovarian carcinoma: a Taiwanese Gynecologic Oncology group study with long-term follow-up. Gynecol Oncol. 2006;101:423-428.
- Fukuda T, Sumi T, Teramae M, et al. Pegylated liposomal doxorubicin for platinum-resistant or refractory Müllerian carcinoma (epithelial ovarian carcinoma, primary carcinoma of fallopian tube and peritoneal carcinoma): a single-institutional experience. Oncol Lett. 2013;5:35-38.
- Lawrie TA, Bryant A, Cameron A, et al. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst Rev. 2013: CD006910.